Dichloroacetaldehyde: Properties, Production and Uses

Dichloroacetaldehyde [79-02-7], or 2,2-dichloroethanal, is a chlorinated acetaldehyde with the chemical formula Cl2CHCHO. It is a colorless liquid with a pungent, irritating odor that was produced for the first time in 1868 by F. Paterno by distillation of dichlorodiethyl acetal, CHCl2CH(OC2H5)2, with sulfuric acid.
Table of Contents
1. Physical Properties of Dichloroacetaldehyde
Dichloroacetaldehyde, also known as 2,2-dichloroethanal, is a colorless liquid with a characteristic pungent odor. It has several key physical properties:
- Molecular weight: 112.94 g/mol
- Melting point: -37.6 to -37.4 °C
- Boiling point: 89.2 °C
- Density (25 °C): 1.4113 g/cm³
- Dipole moment (30 °C): 2.36 D
- Solubility: miscible in water, forming a hydrate, and readily soluble in common organic solvents.
Dichloroacetaldehyde readily reacts with water to form a monohydrate (CAS: 16086-14-9), known as 2,2-dichloro-1,1-ethanediol. This crystalline solid exhibits distinct properties:
- Molecular weight: 130.96 g/mol
- Melting point: 35–50 °C
- Boiling point: 85–95 °C at 101.3 kPa
- Density (20 °C): 1.53–1.54 g/cm³
- Vapor pressure: 6.5 kPa at 20 °C, 25 kPa at 50 °C
- Solubility: soluble in polar organic solvents, insoluble in non-polar ones.
- Flash point: Approximately 95 °C
- Ignition temperature: Approximately 605 °C
2. Chemical Reactions of Dichloroacetaldehyde
Dichloroacetaldehyde undergoes spontaneous polymerization upon storage, forming a solid, colorless polymer. This polymer is converted back to its monomeric form upon heating to 120 °C.
Dichloroacetaldehyde exhibits the characteristic reactions of aldehydes, such as oxidation, reduction, and condensation.
The oxidation reaction with chromic acid produces dichloroacetic acid.
Reduction of dichloroacetaldehyde with ethylaluminum yields 2,2-dichloroethanol.

The self-condensation leads to the formation of 2,2,4,4-tetrachloroacetaldol.
The condensation reaction of dichloroacetaldehyde with ethylbenzene gives p,p’-diethyl-1,1-diphenyl-2,2-dichloroethane, an insecticide known as Perthane.

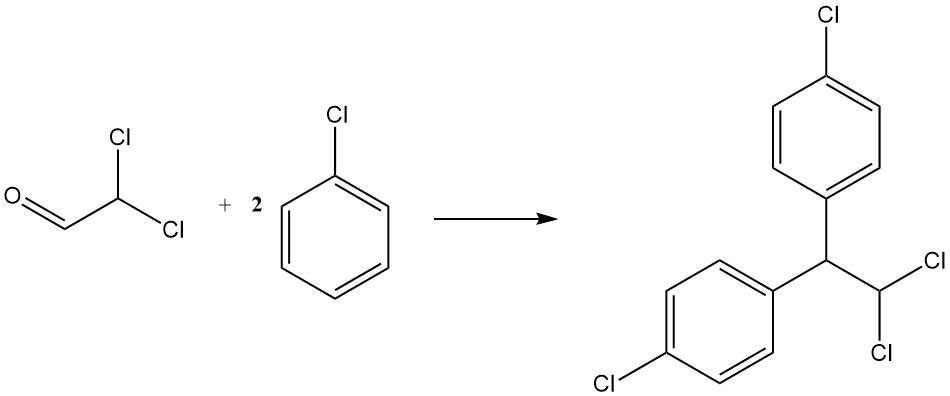
Condensation with chlorobenzene yields p,p’-dichloro-1,1-diphenyl-2,2-dichloroethane, another insecticide known as TDE or DDD. It is represented by the following equation:
CHCl2CHO + 2 C6H5Cl → CHCl2CH(C6H4Cl)2 + H2O
3. Production of Dichloroacetaldehyde
The most important industrial production process for dichloroacetaldehyde is the chlorination of acetaldehyde or paraldehyde. Another method for producing pure dichloroacetaldehyde is the hypochlorination of 1,2-dichloroethylene.
3.1. Chlorination of Acetaldehyde or Paraldehyde
This method generates dichloroacetaldehyde alongside chloroacetaldehyde and trichloroacetaldehyde. High yields are achievable with catalysts like antimony trichloride (83%) or phosphoric acid (>90%). Oxychlorination during the Wacker process for acetaldehyde production can also yield dichloroacetaldehyde as a byproduct.
3.2. Hypochlorination of 1,2-Dichloroethylene
This process offers pure dichloroacetaldehyde, free from impurities like chloral and monochloroacetaldehyde. It involves treating 1,2-dichloroethylene with one mole of chlorine under specific temperature and solvent conditions.
3.3. Chlorination of Ethanol
Controlled chlorination of ethanol in the presence of nickel(II) chloride can primarily yield dichloroacetaldehyde, along with some chloral and monochloroacetaldehyde. The resulting hemiacetal can be directly used without isolating pure dichloroacetaldehyde or its hydrate.

4. Uses of Dichloroacetaldehyde
Dichloroacetaldehyde and its acetals are used in the pharmaceutical industry for the production of trichloromethiazid, a diuretic used to treat conditions like high blood pressure and edema, and mitotane (1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl)ethane), a cytostatic agent used in the treatment of specific adrenal gland cancers.
It is also used in the production of insecticides like Perthane and TDE or DDD.
5. Toxicology of Dichloroacetaldehyde
Dichloroacetaldehyde poses potential respiratory irritation, although formal toxicity testing data is absent. As a precautionary measure, it should be handled carefully.
6. Dichloroacetaldehyde Polymers
6.1. Hexachloroparaldehyde
- Formula: C6H6Cl6O3
- Molar mass: 338.83 g/mol
- Appearance: Colorless crystals
- Melting point: 131-132 °C
- Boiling point: 210–220 °C (decomposition)
Hexachloroparaldehyde is produced by the reaction of dichloroacetaldehyde with Lewis acids like antimony trichloride, iron(III) chloride, or boron trifluoride or by direct chlorination of paraldehyde with 6–7 mol of chlorine at 35 °C under anhydrous conditions.
It forms dichloroacetaldehyde hydrate in the presence of aqueous acids and decomposes to dichloroacetaldehyde at elevated temperatures.
6.2. Polydichloroacetaldehydes
- Formula: (C2H2Cl2O)n
- Solubility: variable depending on polymerization degree, generally soluble in common organic solvents.
The crystalline forms of polydichloroacetaldehydes is produced by treating dichloroacetaldehyde at -78 °C with organometallic compounds like triethylaluminum, and the amorphous forms are prepared using Lewis acids at temperatures below 0 °C.
These polymers are soluble in concentrated sulfuric acid at 100 °C with discoloration and in dimethylformamide. They can form copolymers with other aldehydes.
Reference
- Chloroacetaldehydes; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a06_527.pub2
