Benzenesulfonic Acid: Properties, Production and Uses

What is Benzenesulfonic Acid?
Benzenesulfonic acid is the simplest aromatic sulfonic acid, with the formula C6H5SO3H. It appears as fine, delicate needles or large plates.
Benzenesulfonic acid was first synthesized, together with diphenyl sulfone, by E. Mitscherlich in 1834 by the reaction of benzene with fuming sulfuric acid. Until the early 1960s, benzenesulfonic acid was used mainly in the manufacture of phenol.
Table of Contents
1. Physical Properties of Benzenesulfonic Acid
Benzenesulfonic acid crystallizes from aqueous solution as a deliquescent hydrate with 1.5 molecules of water per molecule of benzenesulfonic acid. This form melts at a range of 43–44 °C. A monohydrate (one water molecule per acid molecule) also exists, melting at 45–46 °C.
The removal of water yields anhydrous benzenesulfonic acid. This form possesses a higher melting point (65–66 °C) and can be distilled without decomposition at 171–172 °C under low pressure (0.13 mbar).
Benzenesulfonic acid is highly soluble in water and alcohols, is slightly soluble in benzene, and is insoluble in diethyl ether and carbon disulfide.
The dielectric constant of benzenesulfonic acid in an aqueous solution is 0.2.
Important physical properties of benzenesulfonic acid are listed in the Table 1.
| Property | Value |
|---|---|
| CAS Number | [98-11-3] |
| Chemical Formula | C6H6SO3 |
| Molecular Mass | 158.17 g/mol |
| Melting Point | 51 °C |
| Boiling Point | 190 °C |
| Density | 1.3 g/cm3 |
| pKa | -2.8 |
| Vapor Density | 5.5 |
| Flash Point | 113 °C |
2. Chemical Reactions of Benzenesulfonic Acid
Benzenesulfonic acid exhibits typical reactions of a strong aromatic sulfonic acid. Acid hydrolysis at 175 °C yields benzene and sulfuric acid.

Further sulfonation with fuming sulfuric acid produces 1,3-benzenedisulfonic acid, which can be converted to 1,3,5-benzenetrisulfonic acid and diphenyl sulfone disulfonic acid.

Benzenesulfonic acid undergoes a Friedel-Crafts-type reaction with benzene to form diphenyl sulfone.
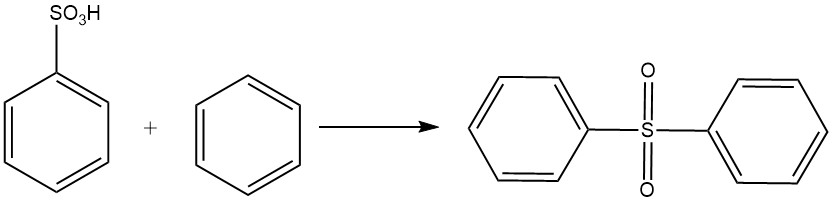
Benzenesulfonic acid reacts with alkali sodium hydroxide at 320-350 °C to produce sodium phenolate according to the following equation:
C6H5SO3Na + 2 NaOH → C6H5ONa + Na2SO3 + H2O
This reaction formed the basis of the first industrial production of phenol.

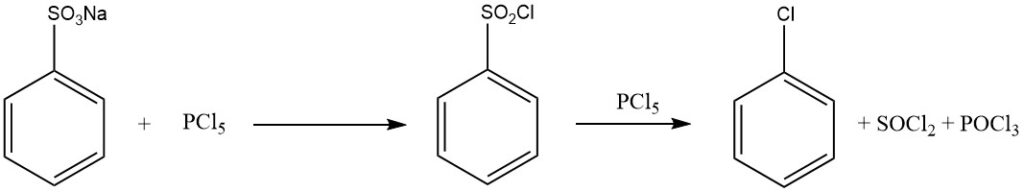
Treating benzenesulfonic acid with phosphorus halogenides (PCl5, PBr5), chlorosulfuric acid, thionyl chloride, or phosgene yields sulfonyl halides:
C6H5SO2OH + PCl5 → C6H5SO2Cl + POCl3 + HCl
When an excess of PCl5 is used, chlorobenzene is formed according to:
C6H5SO2Cl + PCl5 → C6H5Cl + SOCl2 + POCl3

Benzenesulfonic esters are produced by the reaction of sulfonic acid or sulfonyl chlorides with alcohols; for example, methyl benzenesulphonate from methanol, ethyl benzenesulphonate from ethanol and isopropyl benzenesulfonate from isopropanol.
3. Production of Benzenesulfonic Acid
Benzenesulfonic acid is produced by an exothermic reaction between benzene and sulfuric acid, as described by the equation:
C6H6 + H2SO4 → C6H5SO3H + H2O

The reaction reaches equilibrium at a specific sulfuric acid concentration between 74 and 78%, which depends on temperature and water formation.
A significant portion of the sulfuric acid, at around 45% in the traditional process, remains unused, acting as a solvent and diluent. This excess helps minimize the formation of byproducts like diphenyl sulfone. In industrial settings, calcium carbonate is used to remove the excess acid.
Several methods have been implemented industrially to reduce excess sulfuric acid, all leading to a slight increase in sulfone formation by:
- Replacing sulfuric acid with oleum (fuming sulfuric acid) or pure sulfur trioxide.
- Extracting benzenesulfonic acid from the reaction mixture using benzene.
- Continuously removing water formed during the reaction using benzene by azeotropic distillation.
The classical process is no longer commercially relevant because it was connected to phenol production (the use of sulfite from phenol production to neutralize the benzenesulfonic acid).
3.1. Production of Benzenesulfonic Acid by Continuous Sulfonation with Oleum (Monsanto Process)
This process involves simultaneously feeding benzene and oleum into a cascade of six sulfonation vessels equipped with stirrers. The first two vessels are cooled, while the others are heated. Optimal temperature control ensures complete reaction by the time the mixture exits the final vessel.
Excess sulfuric acid is neutralized with sodium sulfite or sodium hydroxide solution, leading to sodium sulfate precipitation. The solution containing sodium benzenesulfonate is concentrated, causing further precipitation of sodium sulfate.
After separation, the benzenesulfonic acid can be directly converted to phenol or dried. This process typically uses oleum with 35.6% sulfur trioxide and maintains temperatures between 70–80 °C and 110 °C in the first and last vessels, respectively. Around 1% of the benzene is converted to diphenyl sulfone.
3.2. Production of Benzenesulfonic Acid by Continuous Extraction Process
This method involves vigorously stirring excess benzene and sulfur trioxide introduced separately into the bottom of a vessel. A benzene layer saturated with benzenesulfonic acid gathers at the top and overflows into a second vessel.
Here, the benzenesulfonic acid is extracted from the benzene using continuous washing with water or sodium hydroxide solution. The separated benzene layer is then dried and recycled back to the reaction vessel.
This process achieves a sulfuric acid consumption of 1260 kg per 1000 kg of converted benzene, with diphenyl sulfone formation below 2%.
3.3. Production of Benzenesulfonic Acid by Azeotropic Removal of Reaction Water
This process involves heating sulfuric acid (around 79% concentration) to 170 °C in a vessel. Finely divided benzene vapor is introduced through a perforated plate at the bottom. A portion of the excess benzene vapor undergoes sulfonation, while the remaining benzene continuously removes the formed water via azeotropic distillation.
The benzene-water vapor mixture exiting the vessel is condensed, and the separated benzene is recycled back to the evaporator. Continuous operation yields a final product containing approximately 80.2% benzenesulfonic acid and 14.3% sulfuric acid.
Batch operations can produce a product with 93.1% benzenesulfonic acid and 4.8% sulfuric acid. Adding sodium benzenesulfonate to the reaction mixture can further reduce sulfone formation below 2% .
4. Uses of Benzenesulfonic Acid
Benzenesulfonic acid is used for diverse applications in various industries, as follows:
Phenol production: Benzenesulfonic acid is used to produce phenol by fusion with sodium hydroxide or hydrolysis of its salts, typically the sodium salt.
Surfactant synthesis: Benzenesulfonic acid is a key component in the creation of surfactants, particularly when combined with metal or amine salts. Salts of benzenesulfonic acid, such as sodium benzenesulfonate (Ludigol) and monoethanolamine benzenesulfonate, are employed as surfactants in laundry detergent formulations.
Pharmaceutical drug synthesis: Benzenesulfonic acid is involved in pharmaceutical drug synthesis, where these drugs are produced as benzenesulfonate salts. These salts are recognized by the International Nonproprietary Name as besilates or the United States Adopted Name as besylates.
Acid catalyst: The acidic nature of benzenesulfonic acid makes it valuable as an acid catalyst in various chemical reactions.
Dye standardization: The sodium salt of benzenesulfonic acid plays a role in standardizing dyes, ensuring the accuracy of coloration processes.
Surfactant-enhanced oil recovery (SEOR): Benzenesulfonic acid is essential in SEOR, a technique that involves the use of surfactants to facilitate the extraction of oil from reservoirs, also known as surfactant flushing.
5. Toxicology of Benzenesulfonic Acid
Benzenesulfonic acid presents potential health risks from exposure by inhalation and ingestion.
Inhalation can cause respiratory irritation, characterized by coughing, a sore throat, shortness of breath, and a burning sensation in the lungs. Headaches and nausea may also accompany these symptoms.
Skin contact with the acid leads to irritation and burns, with redness, burning pain, and potential blistering.
Similarly, eye exposure can cause severe burns and permanent vision damage.
Ingestion of benzenesulfonic acid results in irritation and burning of the throat and chest, along with abdominal pain and potentially even shock or collapse.
Acute toxicity data includes:
- Oral LD50 (rats): 890 uL/kg
- Skin LDLo (cats): 10 mg/kg
- Oral LD50 (birds): 75 mg/kg
Exposure to benzenesulfonic acid can lead to more serious health problems. The corrosive nature of the acid can cause burns on the skin and eyes. Inhaling the acid can inflame the lungs, a condition known as toxic pneumonitis.
While generally not considered to cause long-term health effects, caution is still advised. Contact with heated vapors or the sublimated form (solids transitioning directly to gas) can irritate the upper respiratory tract, eyes, and skin.
Animal studies support the potential hazards of benzenesulfonic acid exposure. Guinea pigs developed severe skin irritation upon contact, and rabbit eye tests indicated significant injury.
References
- Benzenesulfonic Acids and Their Derivatives; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a03_507
- BENZENESULFONIC ACID – ILO
- https://www.acs.org/molecule-of-the-week/archive/b/benzenesulfonic-acid.html
- https://en.wikipedia.org/wiki/Benzenesulfonic_acid
- Benzenesulfonic Acid (Surfactant)
