Ammonium Chloride: Properties, Production and Uses

Ammonium chloride is a chemical compound with the formula NH4Cl. It exists as a white crystalline solid with a characteristic salty taste and is commonly known as sal ammoniac.
Ammonium chloride is naturally found in volcanic material but its extraction from natural sources holds minimal importance. The industrial production of NH4Cl is closely linked to the advancement of the soda industry and the large-scale synthesis of synthetic NH3.
Initially intended for use as a fertilizer, ammonium chloride now finds applications in diverse fields. Its production processes are of particular interest to chemical engineering due to their historical significance as an early example of chemical processing including all three phases: solid, liquid, and gas.
Table of Contents
1. Properties of Ammonium Chloride
Ammonium chloride molar mass is 53.49 g/mol and it is a solid with a specific density of 1.530 at 20 °C. Its average specific heat, cp, between 298 K and 372 K, is measured at 1.63 kJ/kg. Ammonium chloride has two reversible crystallin forms, with the transformation between them occurring at 457.6 K (184.5°C).
The stable form at room temperature is the α form, while the β-NH4Cl (cubic; NaCl type) melts at 793.2 K under 3.45 MPa and sublimes at atmospheric pressure. At lower temperatures, NH4Cl is relatively volatile and dissociates into NH3 and HCl.
The solubility of NH4Cl in water increases with rising temperature. The integral heat of solution to saturation is +15.7 kJ/mol, and the differential heat of solution at saturation is +15.2 kJ/mol.
The addition of ammonia can enhance the solubility in water, while the presence of NaCl tends to precipitate NH4Cl from ammoniacal solutions. NH4Cl also shows weak hygroscopic properties.
Moisture may cause product caking, and it is uncertain if moisture contents lower than 0.1 wt % alone are responsible for ammonium chloride caking. Sublimation could be another potential explanation for this phenomenon.
Ammonium chloride is highly soluble in liquid NH3 but virtually insoluble in acetone and pyridine. At 292.7 K, it exhibits solubility of 3.24 wt % in methanol and 0.64 wt % in ethanol.
The crystal form obtained from aqueous solutions of NH4Cl can be influenced by other substances, which has been exploited to produce large crystals and assists in identifying impurities during NH4Cl production.
2. Production of Ammonium Chloride
Commercial production of ammonium chloride involves two primary processes:
1. Modified Solvay Process (Ammonia – Soda Ash Process or ASAP): This is one of the widely used methods for commercial NH4Cl production. It involves the reaction between ammonia and soda ash (sodium carbonate) to produce ammonium bicarbonate, which is then converted into ammonium chloride by heating.
2. Direct Reaction between HCl and NH3: In this process, ammonium chloride is directly formed by the reaction between hydrochloric acid and ammonia.
Additionally, there is a third process known as the reaction of reciprocal pairs of salts, which is still of interest from a scientific perspective, but it has not yet been commercially applied for ammonium chloride production.
2.1. Modified Solvay (Ammonia – Soda Ash) Process
In the Solvay process, the production of ammonium chloride and sodium carbonate is achieved by a series of reactions. Ammonia and carbon dioxide are dissolved in an aqueous solution of sodium chloride, resulting in the formation of sparingly soluble sodium bicarbonate.
This sodium bicarbonate is then heated (calcined) to yield sodium carbonate. Ammonia is recovered from the remaining liquid (mother liquor) by reacting it with lime, which also generates calcium chloride.
The primary sources of lime and carbon dioxide are limestone. The overall reaction involves the conversion of rock salt and limestone into sodium carbonate and calcium chloride as byproducts.
To compensate for plant losses, only the necessary amounts of water, carbon dioxide, and ammonia are added. The choice of feedstock and the resulting products determine the location of a Solvay plant, making these plants largely independent units.
The modified Solvay process, known as the ammonium chloride – soda ash process, is an early example of integrated industrial production involving two substances. In this modified process, ammonium chloride is additionally precipitated from the mother liquor. The overall reaction for this process is:
2 NH3 + CO2 + H2O + 2 NaCl → 2 NH4Cl + Na2CO3
The quantities of ammonium chloride and soda ash produced are nearly equal: two moles (107 g) of NH4Cl are generated for each mole (106 g) of Na2CO3. The modified process necessitates external NH3 and CO2 and must be integrated into a system of interconnected plants.
Furthermore, the treatment of ammonia-containing waste gases is carried out for environmental purposes, rather than merely to reduce NH3 and CO2 losses. The energy balance in the modified process differs significantly from that in the traditional Solvay process due to the distinct feedstocks and products involved.
2.1.1. Process Description
In the manufacturing process of NH4Cl and Na2CO3, a continuously operated recycle process is used, with the concentrations adjusted based on whether ammonium chloride or sodium carbonate is the primary product. It was initially developed by BASF this process focuses on NH4Cl as the primary product (Figure 1).
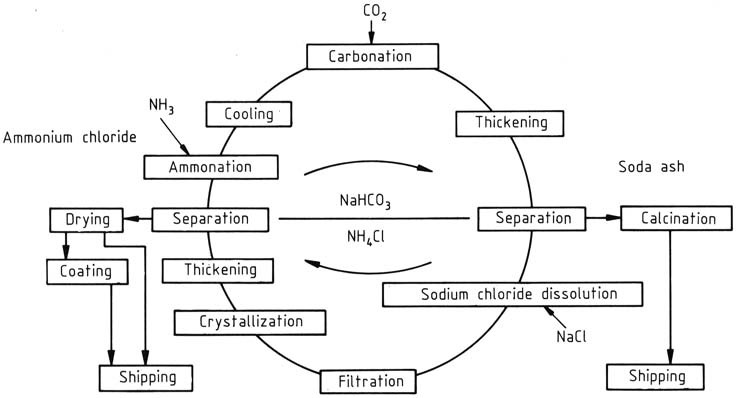
The cycle commences with the addition of ammonia and carbon dioxide to an aqueous recycle solution. Ammonium bicarbonate formed further reacts with sodium chloride in the solution, leading to the generation of ammonium chloride and sparingly soluble sodium bicarbonate. The latter is separated and washed using a centrifuge and subsequently calcined to produce sodium carbonate.
Heating the remaining sodium bicarbonate in the mother liquor to 337.2 K decomposes it. The highly soluble sodium carbonate does not interfere with the crystallization of ammonium chloride.
The high temperature of the solution is used to dissolve rock salt feedstock rapidly, introduced through mixing tanks, with the quantity adjusted based on the material balance over the entire cycle.
Insoluble impurities, such as sulfates, iron, calcium, and magnesium salts, are filtered out. The disposal of residues, an increasing concern, is being addressed by using purer salt as raw material instead of rock salt.
Ammonium chloride is recovered by two-stage crystallization using indirect cooling. The resulting suspension from the crystallizer is thickened in hydrocyclones, and the solid is separated and washed in a centrifuge.
The solid with around 5 – 7 wt % water content is then dried in a current drier to reduce moisture content to less than 0.1%. The final product may receive an anticaking agent coating. Following ammonium chloride removal, the cycle is completed, and the mother liquor can be reused.
Asahi Glass has developed an alternative process that produces soda ash as the primary product. In this process, ammonia is added to the mother liquor before ammonium chloride crystallization. Crushed raw salt may be added with or without washing and is dissolved in the solution.
Cooling the solution below 20 °C causes ammonium chloride crystallization. The crystals are separated using a centrifugal separator, and the mother liquor is recycled to the carbonation section. Ammonium chloride crystals are dried to reduce moisture content to less than 0.3% in a rotary or fluidized bed drier. The size of the crystals can be controlled for various applications.
In the carbonation section, the solution is passed into a carbonating tower, where sodium bicarbonate precipitates by reaction with carbon dioxide at 30 – 40 °C.
The resulting slurry is separated into sodium bicarbonate crystals and the mother liquor. The mother liquor is directed to the ammonia absorption section. The separated crystals are calcined and transformed into dense soda ash.
The Asahi Glass process includes all the steps of the BASF process shown in Figure 1 with a different sequence. Ammonia is added after the separation of sodium bicarbonate. The heat released during ammonia addition provides the energy needed to decompose the remaining sodium bicarbonate.
While energy savings are observed due to this approach, the higher energy demand in the crystallization stage offsets some of the benefits, as ammonium chloride is more soluble in ammonia-rich solutions.
The process may include a washing step for the rock salt feed before solution to eliminate the need for filtration before crystallization. With or without washing, this process maximizes the utilization of raw salt, an important consideration in Japan where salt must be imported.
2.1.2. Pollution problems
Pollution problems related to the operation of the plants are not severe and can be effectively managed by careful operation. With proper measures, the production of wastewater streams can be significantly reduced, if not entirely eliminated. However, any wastewater that is produced due to its high ammonia content will require appropriate treatment.
To address gaseous emissions, a practical solution involves scrubbing waste gases with hydrochloric acid, resulting in the production of additional ammonium chloride.
Typically, high ammonia conversions of around 98% and rock salt conversions of approximately 95% are achieved, though the exact values may vary depending on the specific process type and the implemented environmental measures. This approach helps minimize gaseous emissions and contributes to the overall environmental sustainability of the manufacturing process.
2.1.3. Grades and Capacity
As of 1997, after several production plants were closed due to reduced market demand, the estimated capacity for NH4Cl production by the modified Solvay process in the western hemisphere and Japan was around 100,000 tons per year.
In Asia, the production of a granular product with over 97% NH4Cl content is common. This fertilizer-grade NH4Cl typically contains more than 25 wt % ammonia nitrogen. These plants primarily focus on soda ash production, with NH4Cl being a byproduct.
However, if the primary interest is in NH4Cl production, the process can be tailored to achieve higher purities. Purity levels exceeding 99.7 wt % NH4Cl, with less than 0.25 wt % NaCl and less than 3 ppm Fe, can be achieved, which is suitable for most industrial purposes.
For special applications requiring reagent-grade NH4Cl, large-scale production is possible using specific operating techniques without the need for additional processing steps.
Reagent-grade ammonium chloride with less than 0.01 wt % NaCl and meeting the ACS (American Chemical Society) specification can be obtained. This higher purity NH4Cl is suitable for applications that demand exceptional quality and precision in chemical processes.
2.2. Direct Reaction Between HCl and NH3
The synthesis of NH4Cl from HCl and NH3 can be economically advantageous, particularly when HCl is available as a feedstock at low or no cost.
The Engeclor process, developed by the Brazilian company Engeclor, carries out the reaction in an aqueous solution. Ammonia is introduced into the conical section of a saturator, while HCl, diluted with air, is passed into the NH4Cl suspension.
The reaction takes place at 353 K under reduced pressure, with an excess of NH3 to maintain a pH of 8. The resulting suspension is withdrawn from the base of the saturator, thickened using hydrocyclones, and NH4Cl is separated from the mixture in a centrifuge and then dried.
The mother liquor is recycled back to the saturator. To control emissions, waste gases from the saturator are scrubbed with water.
Alternate schemes have been proposed where gaseous feedstocks are introduced into nonaqueous solvents. In these cases, the heat of reaction (-176 kJ/mol) is removed by evaporating the solvents, which are then condensed.
Some fluidized bed processes have also been described for this purpose. To prevent aerosol formation, carbon dioxide is suggested as a carrier gas in fluidized beds.
The estimated global annual production of NH4Cl by the HCl-NH3 process was 50,000 tons in 1997. Ammonium chloride produced using this method contains less than 0.1 wt % NaCl.
The metal content, especially the heavy metal content, may vary depending on the specific plant. Generally, the metal levels in NH4Cl produced by this process are higher than those in the modified Solvay process.
2.3. Reaction of Reciprocal Pairs of Salts
The reaction of reciprocal pairs of salts remains an method of interest. Some suitable pairs of salts include (NH4)2SO4 – KCl, which results in the formation of NH4Cl – K2SO4 and (NH4)2SO4 – NaCl, which leads to the production of NH4Cl – Na2SO4.
The challenge with these salt pairs is the lower purity of NH4Cl due to the relatively high sulfate content. NH4Cl and KNO3 can be obtained by the reaction of NH4NO3 and KCl. High-purity KNO3 can be used in the production of explosives, while NH4Cl with a minimum purity of 96% is suitable as a fertilizer.
Additionally, the preparation of NaNO3 and NH4Cl from NaCl and NH4NO3 has been a subject of investigation. This process explores the possibility of obtaining these two salts from their respective precursor salts.
3. Uses of Ammonium Chloride
Ammonium chloride finds extensive agricultural and industrial applications:
3.1. Agricultural Use
In Japan, China, and Southeast Asia, ammonium chloride is widely used as a highly effective nitrogen fertilizer for paddy and upland rice, wheat, and various other crops.
In Japan, a significant portion of the annual NH4Cl production is used for high-grade compound fertilizers, such as chloro-ammonium phosphate, chloro-potash-ammonium phosphate, magnesia-chloro-potash-ammonium phosphate, and nitrogen-potash blends. However, its usage as a fertilizer is limited due to its acidity and high chlorine content.
3.2. Industrial Use
Technical-grade ammonium chloride is employed in various industrial applications:
- Solid Electrolytes: It serves as a vital component in dry cell batteries.
- Quarrying Explosives: A fine form of ammonium chloride is used as a component in explosives for quarrying purposes.
- Hardeners for Adhesives: It acts as a hardening agent in formaldehyde-based adhesives.
- Etching Solutions: Ammonium chloride, along with other chemicals, is used in the production of printed circuit boards.
- Fluxes in Plating: It is a component, along with zinc chloride, in fluxes used for tin and zinc plating.
- Photography: It serves as a rapid fixer additive in photography.
- Cleaner Additives: Ammonium chloride is used as an additive in cleaning products.
- Nutrient in Yeast Cultures: It is used as a nutrient in yeast cultures for various applications.
- Tanning: In the tanning industry, ammonium chloride finds applications.
- Refining of Precious Metals: It is used in the refining process of precious metals.
- Textile Printing and Dyeing: Ammonium chloride is utilized in textile printing and dyeing.
- Rubber Industry: It finds applications in the rubber industry.
- Tiles and Bricks: Ammonium chloride can be added to tiles and bricks before firing to control porosity and accelerate the firing process.
3.3. High-Purity Applications
High-purity ammonium chloride is employed in the food and pharmaceutical industries, as well as in specific chemical syntheses.
It’s worth noting that the use of NH4Cl as a hardener for formaldehyde-based adhesives has decreased significantly due to its substitution by chloride-free products.
References
- Ammonium Compounds; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a02_243
Process of making ammonium chloride. – https://patents.google.com/patent/US2133513A/en
FAQ
Yes, ammonium chloride is a salt. It is formed by the reaction of an acid (HCl) and a base (NH3).
NH4Cl is an acid. It is formed by the reaction of strong acid, namely hydrochloric acid (HCl) with a weak base, namely ammonium hydroxide (NH4OH).
Ammonium chloride has diverse applications:
– It serves as an effective nitrogen fertilizer for various crops, including rice, wheat, and more.
– In the industrial sector, it is used in dry cell batteries, explosives, adhesives, printed circuit board manufacturing, and plating processes.
– It finds usage in photography, cleaning additives, yeast cultures, tanning, refining precious metals, textile printing, and the rubber industry.
– High-purity ammonium chloride is used in food, pharmaceuticals, and specific chemical syntheses.
Ammonium chloride can be produced by various methods, one of which involves reacting ammonia gas (NH3) with hydrochloric acid (HCl) in an aqueous solution. The resulting ammonium chloride is then separated and dried for use.
Ammonium chloride should be disposed of in accordance with local regulations and guidelines for chemical waste disposal. It is essential to follow proper procedures to avoid environmental contamination.
When heated, ammonium chloride undergoes sublimation, meaning it directly transitions from a solid to a gas without melting. It decomposes into ammonia (NH3) and hydrogen chloride (HCl) gases.
Ammonium chloride appears as white crystalline solid or powder with a granular texture. It is odorless and has a distinctive salty taste.
