Pyrogallol: production, reactions and uses

Pyrogallol, also known as 1,2,3-trihydroxybenzene, is a white crystalline solid with the formula C6H6O3. Pyrogallol exists in the form of colorless needles or leaflets, which gradually change color to dark gray upon exposure to air or light.
It is a polyphenol that was initially discovered by SCHEELE in 1786 by performing dry distillation of gallic acid (3,4,5-trihydroxybenzoic acid).
Pyrogallol derivatives are naturally found in several compounds, including tannin, anthocyanin, and alkaloids.
Table of Contents
1. Production of Pyrogallol
Pyrogallol is commercially produced by decarboxylation of gallic acid, which is prepared by hydrolysis of tannin.
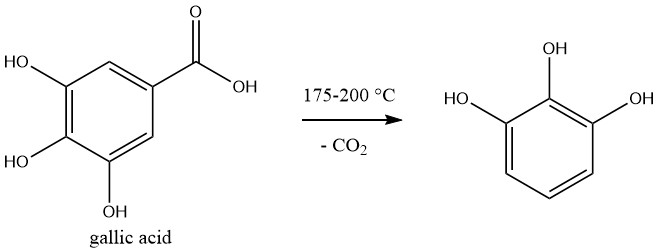
The reaction is carried out in a batchwise. A 50% aqueous solution of gallic acid is heated to 175-200°C in an autoclave, and the pressure increases to 1.2 MPa during the reaction.
Upon completion of the evolution of carbon dioxide, the solution is cooled. The reaction proceeds quantitatively.
After decolorization of the reaction mixture with charcoal, a crude product is obtained, which is purified by sublimation or vacuum distillation.
The cost of obtaining pyrogallol is relatively high, as the starting material gallic acid is derived from a natural product that has limited availability. Consequently, alternative methods for producing gallic acid have been sought.
Base-catalyzed condensation of trimethyl propane-1,2,3-tricarboxylate with the dimethyl ketal of mesoxalic acid, followed by hydrolysis and decarboxylation, affords gallic acid in 74% yield. The reaction of the ketal and glutaric ester yields pyrogallol without the generation of gallic acid.
Various other methods have also been developed for producing pyrogallol, such as the oxidation of resorcinol with hydrogen peroxide, hydrolysis of 2,6-diamino-4-butylphenol, demethylation of 4-substituted 2,6-dimethoxyphenols, oxidation of 2,6-dimethylphenol, hydrolysis of 2,2,6,6-tetrachlorocyclohexanone, deoximation of 1,2,3-cyclohexanetrion-1,3-dioxime, and dehydrogenation of 1,2,3-trihydroxycyclohexane.
2. Chemical Reactions of Pyrogallol
Pyrogallol, a polyhydroxybenzene, is considered the most potent reducing agent. Its aqueous solution rapidly absorbs gaseous oxygen and precipitates a dark brown substance.
Pyrogallol has been widely employed for the quantitative determination of oxygen owing to this property.
The chemical behavior of pyrogallol is comparable to that of phenols. Mono-, di-, and trisubstituted products can be synthesized through traditional methods such as esterification and etherification of the hydroxy groups.
Upon heating with aqueous potassium bicarbonate, pyrogallol predominantly yields pyrogallol-4-carboxylic acid (2,3,4-trihydroxybenzoic acid), with gallic acid as a byproduct.
Pyrogallol can undergo formylation, acylation, and Mannich reaction to form 4-substituted cyclohexenones as the primary product.
Bromination with bromine eventually results in 1,2,6,6-tetrabromocyclohexene-3,4,5-trione. Reaction with phosgene yields pyrogallol carbonate, and with thionylbromide produces 4,5,6-tribromopyrogallol.
The quantitative analysis of heavy metal ions is accomplished via the highly sensitive color reactions of pyrogallol aqueous solution with them. Precious metal ions (e.g., Ag+, Au2+, Hg2+) are reduced to elemental metals.
A catalyst for alternating polymerization of carbon dioxide and propylene oxide is created by a complex formed from diethylzinc and pyrogallol.
3. Uses of Pyrogallol
Pyrogallol is a versatile compound that finds utility in photography, lithography, and hair dyes, and is employed as an antioxidant and stabilizer in various applications.
The use of pyrogallol in the domain of cosmetics and medicines has decreased in recent times, attributed to its significant toxicity.
Reference
- Phenol Derivatives; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a19_313
