Levulinic Acid: Properties, Reactions, Production and Uses
What is Levulinic Acid?
Levulinic acid, also known as 4-oxopentanoic acid or γ-ketovaleric acid, is the simplest and most important γ-oxocarboxylic acid with the formula C5H8O3. It is a white crystalline solid that is soluble in water and polar organic solvents.
Levulinic acid was first described and named by Grote and Tollens in 1875, but it wasn’t until 1960 that it gained commercial importance.
Table of Contents
1. Physical Properties of Levulinic Acid
Levulinic acid forms colorless crystals that are readily soluble in water, ethanol, and diethyl ether.
Important physical properties of levulinic acid are given in the table below.
| Property | Value |
|---|---|
| CAS number | [123-76-2] |
| Chemical formula | H3C-CO-CH2-CH2-COOH |
| Molecular mass | 116.11 g/mol |
| Melting point | 37 °C |
| Boiling point | at (101.31 kPa): 245–246 °C at (1.33 kPa): 137–139 °C |
| Specific density (20 °C) | 1.14 |
| Refractive index (25 °C) | 1.4396 g/cm3 |
| pKa | 4.64 (at 18 °C) |
| Flash point | 138 °C |
2. Reactions of Levulinic Acid
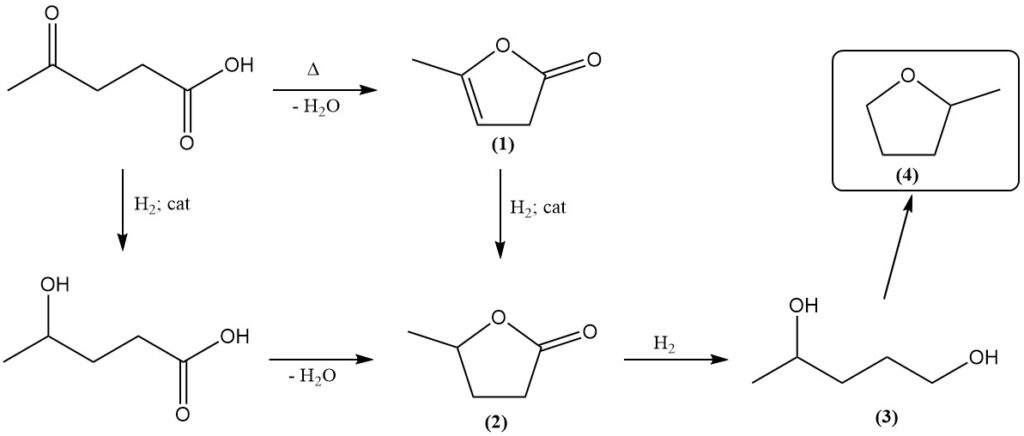
Levulinic acid reacts as both a ketone and a carboxylic acid. Prolonged heating induces dehydration, yielding angelica lactone (1).
Catalytic hydrogenation transforms levulinic acid into γ-valerolactone (2). Different catalysts, including platinum oxide, Raney nickel, copper-chromite, and rhenium-based systems, have been employed. Subsequent hydrogenation yields 1,4-pentanediol (3), a precursor to methyltetrahydrofuran (4).
Condensation reactions with aldehydes occur at the α, β, or δ carbon positions, depending on the reaction conditions.
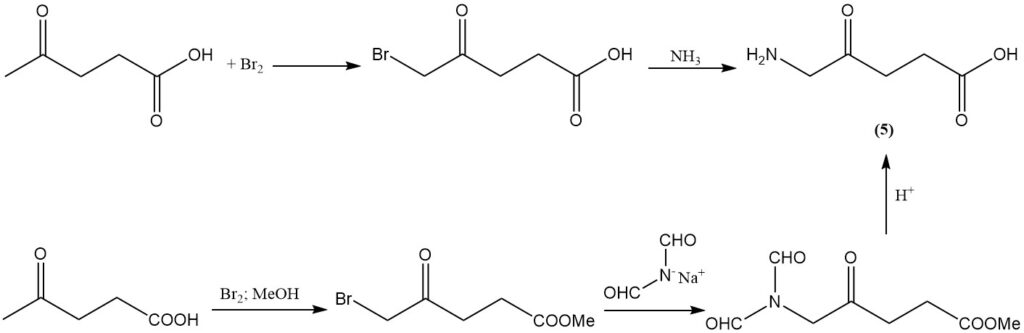
The bromination of levulinic acid with bromine, followed by amination, produces aminolevulinic acid (5), which is an herbicide with broad-spectrum activity. This herbicide can also be produced in high yield by bromination of levulinic acid, followed by reaction with the diformylamide anion, and then acid hydrolysis.
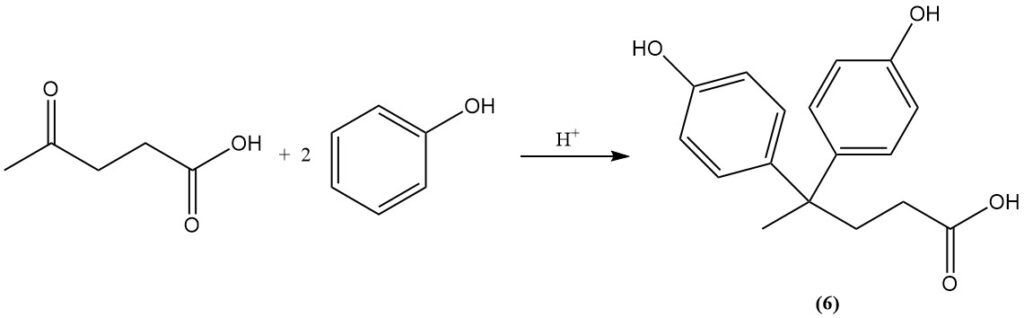
The reaction of levulinic acid with phenol yields diphenolic acid (6), which is a compound used in the production of polymers.
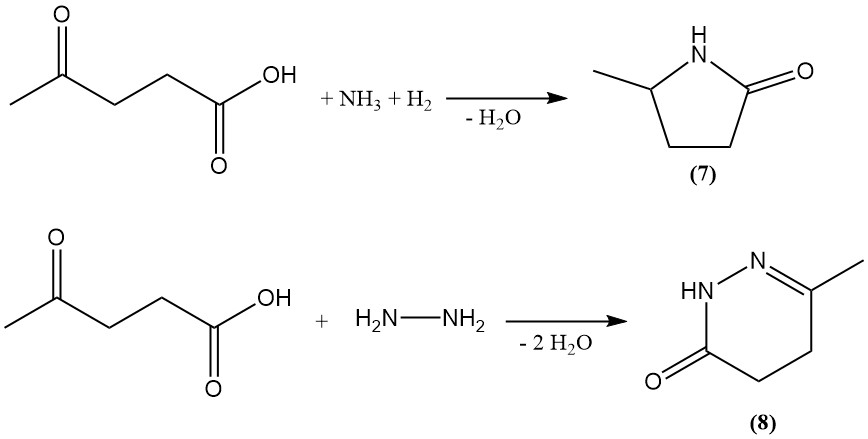
Levulinic acid is used as a precursor for the synthesis of diverse heterocyclic compounds. Reductive amination generates 5-methyl-2-pyrrolidone (7), while reaction with hydrazine produces 6-keto-3-methyl-1,4,5,6-tetrahydropyridazone (8).
Levulinic acid can react with alcohols to form esters, such as ethyl levulinate, which is used in fragrances.
3. Production of Levulinic Acid
Levulinic acid is industrially synthesized from carbohydrate polymers, such as cellulose or starch. These polymers are initially depolymerized into hexose monomers, primarily D-glucose, via acid-catalyzed hydrolysis, followed by enzymatic isomerization of D-glucose to D-fructose.
Subsequent dehydration of D-fructose yields hydroxymethylfurfural, a key intermediate in levulinic acid formation. The classical synthesis uses the direct reaction of D-fructose with hydrochloric acid.
Alternative synthesis pathways include ester hydrolysis, furfuryl alcohol hydrolysis, ketone oxidation, palladium-catalyzed carbonylation of ketones, and nitroalkane alkylation. However, these methods often yield significant byproducts or necessitate costly starting materials.
The Biofine process (Figure 1) represents a notable advancement in levulinic acid production. A two-stage reactor system is employed for levulinic acid synthesis from carbohydrate feedstocks.
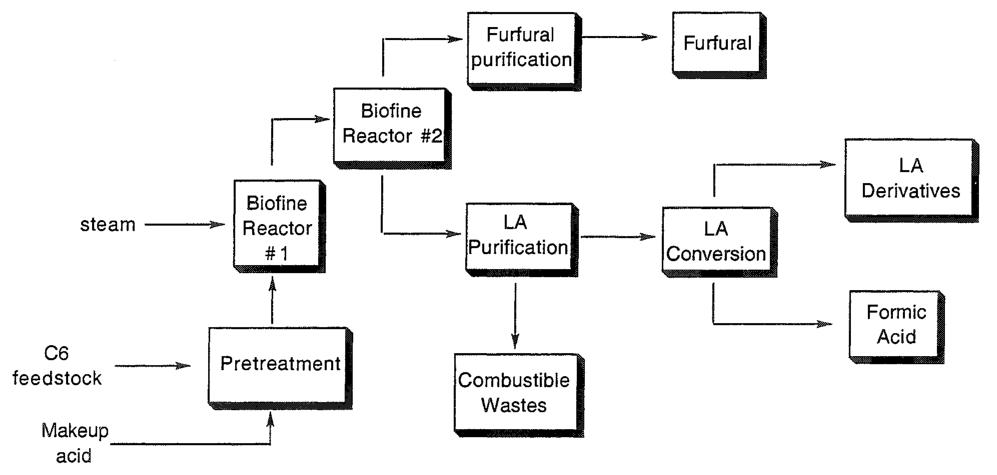
In the initial reactor, carbohydrate hydrolysis occurs at temperatures ranging from 210 to 230 °C for a brief 13 to 25 seconds in the presence of a dilute mineral acid catalyst. This stage produces hydroxymethylfurfural, which is continuously extracted and transferred to the subsequent reactor.
Within the second reactor, hydroxymethylfurfural undergoes further hydrolysis at temperatures between 195 and 215 °C for a duration of 15 to 30 minutes. Levulinic acid is continuously removed from this reactor.
The overall levulinic acid yield, calculated based on the initial hexose content of the carbohydrate feedstock, is approximately 60%. This efficiency is notably high compared to previously reported methods.
This process gives high yields, reduced byproduct formation, and the potential for cost-effective production from diverse cellulosic waste streams. Economic projections indicate substantial energy savings and waste reduction through large-scale implementation of the Biofine process.
Alternatively, levulinic acid can be produced from petrochemical feedstocks. A patented process uses the ozonolysis of unsaturated hydrocarbons to generate levulinic acid, even though the reaction is complex.
4. Uses of Levulinic Acid
Levulinic acid is used as a precursor for the synthesis of pharmaceuticals, plasticizers, and as an auxiliary in electroplating. It is used in the production of aminolevulinic acid (a biodegradable herbicide), gamma-valerolactone (a solvent, fuel additive, and intermediate for other chemicals), and diphenolic acid.
The calcium salt of levulinic acid is used in calcium therapy, and levulinic acid esters are used in the production of cosmetics and fragrances.
5. Toxicology of Levulinic Acid
Levulinic acid shows low acute toxicity. Oral LD50 in rats is determined to be 1850 mg/kg. Dermal LD50 in rats is greater than 2000 mg/kg. Inhalation toxicity data is unavailable.
Levulinic acid is a skin irritant. It causes severe eye damage. It is a skin sensitizer, as indicated by positive results in local lymph node assays.
Levulinic acid does not induce chromosomal aberrations in human lymphocytes or gene mutations in Chinese hamster lung cells in vitro.
Available data on carcinogenicity, reproductive toxicity, and specific target organ toxicity for levulinic acid is insufficient.
Levulinic acid does not contain components identified as endocrine disruptors, according to REACH regulations.
Toxicity, persistence, and degradability data for levulinic acid in the environment are limited.
Levulinic acid is harmful if swallowed, may cause allergic skin reactions, and causes serious eye damage.
References
- Oxocarboxylic Acids; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.a18_313
- https://pubchem.ncbi.nlm.nih.gov/compound/Levulinic-acid
- https://www.aceee.org/files/proceedings/1999/data/papers/SS99_Panel1_Paper60.pdf
- https://www.merckmillipore.com/INTL/en/product/msds/MDA_CHEM-814254
