Ethylbenzene: Properties, Production, Uses and Toxicology
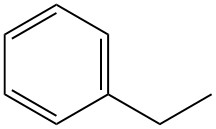
What is ethylbenzene?
Ethylbenzene, also known as phenylethane, is a single-ring alkylaromatic hydrocarbon with the formula C8H10. It is a highly flammable, colorless liquid with a characteristic aromatic odor.
It is used primarily (>99%) as an intermediate in the production of styrene monomer, one of the most significant high-volume commodity chemicals globally.
The commercial-scale production of ethylbenzene began in the 1930s by Dow Chemical (USA) and BASF (Germany). The ethylbenzene/styrene industry remained relatively unimportant until World War II.
The surge in demand for synthetic styrene-butadiene rubber (SBR) during the war years accelerated rapid technological advancements and significant capacity expansion. This wartime effort led to the construction of numerous large-scale factories, and styrene production swiftly evolved into a major industry.
By 1999, global annual production capacity for ethylbenzene had reached nearly 25 million tons. Notably, the 1990s witnessed the most significant capacity growth in Far Eastern countries (excluding Japan), where the fundamental petrochemical industries underwent substantial development and expansion.
Table of Contents
1. Physical Properties of Ethylbenzene
Under normal conditions, ethylbenzene is a colorless liquid with an aromatic odor. It is insoluble in water but soluble in all proportions in ethanol and ethyl ether. Ethylbenzene is an irritant to the skin and eyes and is moderately toxic by ingestion, inhalation, and skin adsorption.
Some physical properties of ethylbenzene are listed in the following table.
| Property | Value |
|---|---|
| CAS number | [100-41-4] |
| Chemical formula | C6H5CH2CH3 |
| Molecular mass | 106.168 g/mol |
| Density |
|
| Melting point | -94.949 °C |
| Boiling point (101.3 kPa) | 136.2 °C |
| Refractive index |
|
| Vapor density | 3.66 |
| Critical pressure | 3609 kPa (36.09 bar) |
| Critical temperature | 344.02 °C |
| Flash point (closed cup) | 15 °C |
| Autoignition temperature | 460 °C |
| Flammability limit |
|
| Latent heat |
|
| Heating value |
|
| Kinematic viscosity |
|
| Surface tension | 28.48 mN/m |
| Specific heat capacity |
|
| Acentric factor | 0.3026 |
| Critical compressibility | 0.263 |
2. Chemical Reactions of Ethylbenzene
The most significant commercial reaction of ethylbenzene is its dehydrogenation to styrene. This reaction occurs at high temperatures (600–660 °C) and typically uses a potassium-promoted iron oxide catalyst with steam as a diluent.
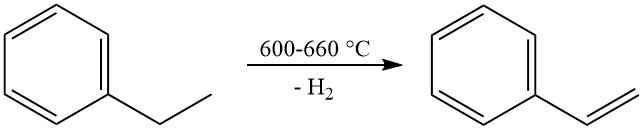
Industrial production achieves styrene selectivities ranging from 90 to 97 mol% with per-pass conversions of 60–70%. The primary side reaction is the dealkylation of ethylbenzene to benzene and toluene.
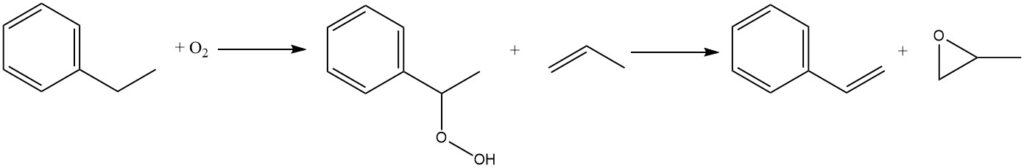
Another reaction gaining commercial importance is the air oxidation of ethylbenzene to ethylbenzene hydroperoxide. It is an uncatalyzed process that occurs in the liquid phase. However, due to the inherent instability of hydroperoxides, exposure to high temperatures must be minimized to reduce the decomposition rate.
The hydroperoxide is subsequently treated with propylene to co-produce styrene and propylene oxide. In 1999, approximately 15% of global ethylbenzene production was directed towards the co-production of styrene monomer and propylene oxide via this route.
Similar to toluene, ethylbenzene can be dealkylated to benzene through catalytic or thermal processes. Also, ethylbenzene undergoes various other reactions characteristic of alkylaromatic compounds, such as alkylation, acylation, nitration, and sulfonation.
3. Industrial Production of Ethylbenzene
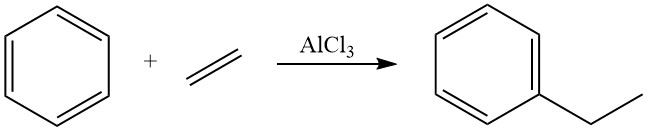
Historically, for several decades, the dominant method for ethylbenzene production was the Friedel-Crafts alkylation of benzene with ethylene using dissolved Lewis acids, primarily aluminum chloride, as catalysts in the liquid phase. This method remains in use for approximately 40% of global ethylbenzene production.
While the aluminum chloride method can be economically competitive, this process generates waste streams that are increasingly expensive to dispose of due to environmental regulations. Also, aluminum chloride is highly corrosive, leading to significant maintenance needs for equipment and piping.
Since the early 1980s, heterogeneous zeolite catalysts have become the preferred technology for new ethylbenzene production plants. The initial zeolite technology operated in the vapor phase, which provided improved process efficiency compared to liquid-phase aluminum chloride.
More recently, liquid-phase zeolite processes have been developed, offering additional flexibility. This technology generates less hazardous waste compared to the aluminum chloride route.
Rising environmental concerns and advancements in zeolite technology have incentivized many producers using aluminum chloride units to retrofit their plants with zeolite technology. Between 1997 and 1999, approximately 106 tons of capacity were converted from aluminum chloride to zeolite processes.
As of 2000, further conversions were in the engineering phase, and the construction of new plants using aluminum chloride technology has virtually ceased in the past decade.
A few plants produce a minor amount of ethylbenzene by superfractionation of mixed C8 aromatic streams.
3.1. Ethylbenzene Production using Aluminum Chloride and Other Lewis Acid Catalysis
For several decades, liquid-phase aluminum chloride processes dominated ethylbenzene production. Several companies, including Dow, BASF, Shell, and Monsanto, developed variations of this technology. Today, approximately 40% of global ethylbenzene production still utilizes AlCl3.
The alkylation of benzene with ethylene is highly exothermic, releasing a significant amount of heat (ΔH = -114 kJ/mol). The use of aluminum chloride as a catalyst in this reaction promotes a fast reaction with near-stoichiometric yields of ethylbenzene.
Other Lewis acids like AlBr3, FeCl3, ZrCl4, and BF3 can also be employed. Processes using AlCl3 often incorporate promoters like ethyl chloride or hydrogen chloride. These promoters reduce the amount of AlCl3 needed for the reaction.
The conventional AlCl3 process (Figure 1) uses three phases in the reactor: aromatic liquid (benzene), ethylene gas, and a red oil catalyst complex phase. The reaction occurs near thermodynamic equilibrium, requiring a single reactor for both alkylation and transalkylation.
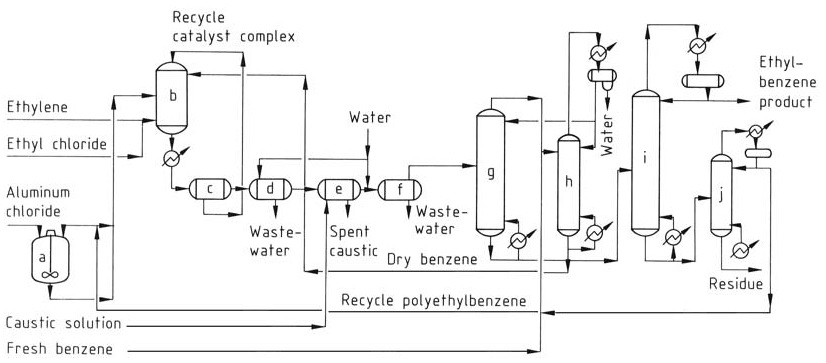
a) Catalyst mix tank; b) Alkylation reactor; c) Settling tank; d) Acid separator; e) Caustic separator; f) Water separator; g) Benzene recovery column; h) Benzene dehydrator column; i) Ethylbenzene recovery column; j) Polyethylbenzene column
The catalyst complex mixture, dry benzene, and recycled polyalkylbenzenes are continuously fed into the reactor. Ethylene and a catalyst promoter (e.g., ethyl chloride) are injected into the reaction mixture.
Operating temperatures are limited to 130 °C to prevent catalyst deactivation and byproduct formation, and sufficient pressure is maintained to keep reactants in the liquid phase.
The improved Monsanto process (Figure 2) offers advantages over conventional AlCl3 processes and has been implemented in many older plants.
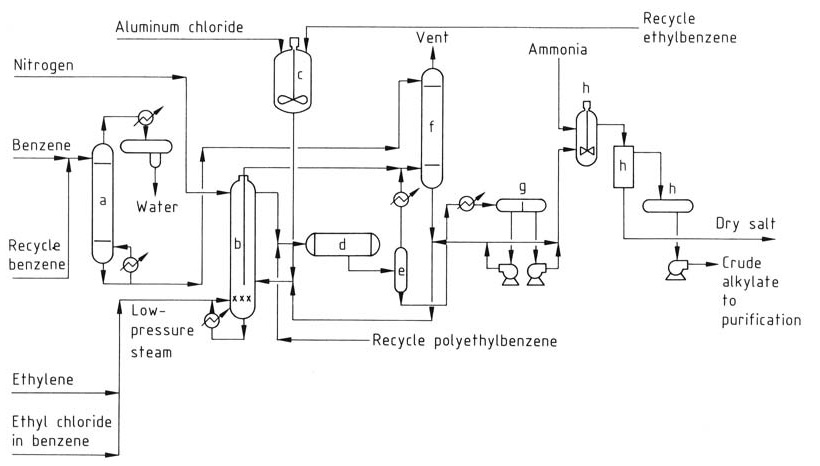
a) Benzene drying column; b) Alkylation reactor; c) Catalyst preparation tank; d) Transalkylator; e) Flash drum; f) Vent gas scrubbing system; g) Decantor; h) Neutralization system
This process operates at higher temperatures (160–180 °C) and uses a homogeneous liquid phase. The higher temperature allows for waste heat recovery as steam. However, a separate transalkylation reactor is required due to the lower catalyst concentration.
By optimizing temperature and ethylene addition, the process significantly reduces the amount of AlCl3 catalyst required, minimizing waste disposal problems and since it operates in a single, homogeneous liquid phase, it eliminates the complex catalyst phase present in traditional methods.
Both conventional and Monsanto processes employ similar purification steps involving multiple distillation columns to separate unconverted benzene, ethylbenzene, and polyalkylbenzene fractions. Organic residue (flux oil) is typically used as fuel.
The Alkar process, developed by UOP, utilized boron trifluoride as a Lewis acid catalyst. While offering advantages like high-purity ethylbenzene product and possibility of using dilute ethylene feedstock, the process is not favorable due to the high maintenance costs and the catalyst sensitivity to even small amount of water.
3.2. Production of Ethylbenzene by Vapor-Phase Alkylation over Zeolites
The Mobil-Badger vapor-phase technology, leveraging Mobil’s ZSM-5 synthetic zeolite catalyst, emerged in the 1970s. This technology has been offered in various configurations.
The first-generation design, commercialized in 1980, performed alkylation and transalkylation in a single reactor, similar to aluminum chloride processes. The latest third-generation technology separates these processes, offering advantages in yield, purity, and capital cost.
The vapor-phase zeolite process is particularly suitable for dilute ethylene streams, such as those derived from fluid catalytic cracking units in refineries.
Until the introduction of liquid-phase zeolite technologies in the 1990s, this process dominated new plant installations due to its ability to avoid the aqueous waste streams generated by aluminum chloride technology.
Mobil-Badger has licensed 31 units since 1980, and the technology remains relevant for dilute ethylene feedstocks.
The fixed-bed ZSM-5 catalyst promotes the same alkylation reaction as other processes. However, ethylene adsorption onto the zeolite’s Brønsted acid sites activates the ethylene molecule, facilitating its bonding with benzene. This mechanism leads to a different byproduct compared to Friedel-Crafts processes.
Carbon steel is the primary construction material due to the absence of highly corrosive components. The third-generation design is depicted in Figure 3.
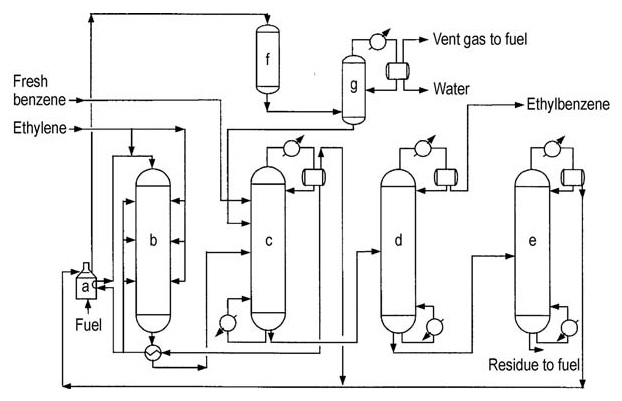
a) Reactor-feed heater; b) Alkylation reactor; c) Benzene recovery column; d) Ethylbenzene recovery column; e) Polyethylenebenzene recovery column; f) Secondary reactor; g) Stabilizer
The alkylation reactor operates at 350–450 °C and 1-3 MPa, allowing for the recovery of most process heat as steam. The reactor uses a multi-bed configuration with a fired heater and heat recovery equipment.
Excess benzene is maintained relative to ethylene. The catalyst gradually deactivates due to coke formation, requiring periodic in-situ regeneration (approximately every 18–24 months), which takes around 36 hours.
Compared to liquid-phase zeolites and Lewis acid catalysts, this technology shows greater tolerance towards water, sulfur, and other potential poisons.
The vaporized reactor effluent undergoes purification. Benzene is recovered via distillation in the first column and recycled. The second column separates ethylbenzene from the bottom stream, which is then directed to a final column for the separation of recyclable alkylbenzene and polyalkylbenzene fractions from the heavy, non-recyclable residue.
This low-viscosity residue, primarily composed of diphenylmethane and diphenylethane, is used as fuel. The separated recyclable higher alkylbenzenes and polyalkylbenzenes are fed to the vapor-phase transalkylator, where they react with excess benzene over a zeolite catalyst.
The transalkylator operates at lower pressure but at a higher temperature compared to the alkylator, promoting dealkylation of higher alkylbenzenes while transalkylating diethylbenzene to ethylbenzene. This capability for dealkylation minimizes overall residue production.
The earlier first- and second-generation processes differed primarily in the location of the recycle polyethylbenzene stream, which led to lower performance compared to the third-generation design.
3.3. Ethylbenzene Production by Liquid-Phase Alkylation over Zeolites
Liquid-phase zeolite technology for ethylbenzene production emerged in the early 1990s. The first commercial plant, operating in Oita, Japan, used ultrastable zeolite Y or zeolite beta catalysts, licensed by ABB Lummus Global and Unocal (later acquired by ABB).
Another process, EBMax from Mobil-Badger, employed Mobil MCM-22 catalyst. By 1999, twelve plants were operational worldwide.
Compared to earlier technologies using polymer-grade ethylene, liquid-phase zeolite processes require less investment and deliver a higher-quality ethylbenzene product.
Vapor-phase technology is now primarily licensed by Mobil-Badger for applications involving dilute ethylene feedstocks.
Liquid-phase processes necessitate wider pore zeolites, compared to ZSM-5 used in vapor-phase processes, to overcome diffusion limitations. Off-site catalyst regeneration is generally recommended due to extended catalyst cycle times, reducing on-site equipment needs and investment costs.
Both major licensors (ABB Lummus and Mobil-Badger) offer similar process flowsheets (Figures 4 and 5).
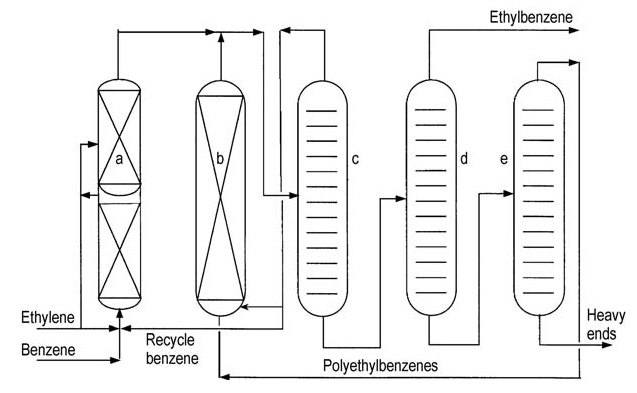
a) Alkylation reactor; b) Transalkylation reactor; c) Benzene column; d) Ethylbenzene column; e) Polyethylbenzene column
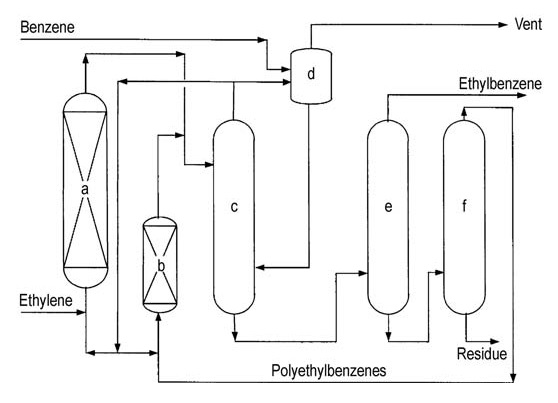
a) Alkylation reactor; b) Transalkylation reactor; c) Benzene column; d) Vent-gas column; e) Ethylbenzene column; f) Polyethylbenzene column
Ethylene is introduced into a multi-stage fixed-bed reactor containing excess benzene. Reactor temperatures are maintained below the critical temperature of benzene (289 °C). High pressures at around 4 MPa are necessary to keep light gases in solution.
Excess benzene is separated from the reactor effluent and recycled back to the alkylation stage.
A series of distillation columns purifies the crude ethylbenzene. Benzene distillation removes excess benzene for recycling. Ethylbenzene distillation separates the desired product, and polyethylbenzene distillation recovers higher alkylbenzenes and polyethylbenzenes from residue.
The recovered higher alkylbenzenes and polyethylbenzenes are fed to a transalkylation reactor for further conversion. The main impurities in the final ethylbenzene can include non-aromatics, toluene, and higher alkylbenzenes, originating from either the feed or production processes.
3.4. Production of Ethylbenzene by Mixed-Phase Zeolite-Based Process
CDTech, a collaboration between ABB Lummus Global and Chemical Research and Licensing, offered a mixed-phase zeolite-based ethylbenzene production process. The first commercial plant began operation in 1994, and by 1999, three such plants were in operation.
The innovative feature of this process is the alkylation reactor, which incorporates a zeolite catalyst in a reactive distillation column.
Both ethylene (gas) and benzene (liquid) are fed to the reactive distillation column. This capability enables the process to use dilute ethylene streams produced during steam cracker distillation.This process can also be adapted for polymer-grade ethylene feedstock.
The process flow is shown in Figure 6.
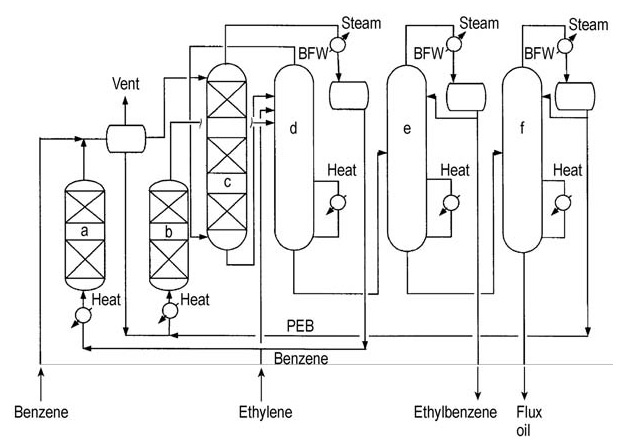
a) Finishing reactor; b) Transalkylator; c) Alkylator; d) Benzene stripper; e) Ethylbenzene column; f) Polyethylbenzene column. BFW = boiler feed water, PEB = polyethylbenzene
The alkylation and benzene stripping occur simultaneously in the same reactive distillation column. Unconverted ethylene and excess benzene are drawn off as overhead products and directed to a finishing reactor for further processing.
The ethylbenzene product is separated from the bottom stream in the overhead product column. The remaining residue is further processed to recover transalkylatable polyethylbenzenes.
The isolated polyethylbenzenes are then sent to a separate liquid-phase transalkylation reactor along with excess benzene. The product from this transalkylation stage is returned to the fractionation train for further purification.
3.5. Separation of Ethylbenzene from Mixed C8 Streams
Less than 1% of global ethylbenzene production originates from the separation of mixed C8 aromatic streams, which typically occurs alongside xylene production from reformate. While adsorption processes like UOP’s EBEX exist, distillation remains the primary method for ethylbenzene recovery from this source.
The difficulty of separating ethylbenzene from these streams necessitates a process known as superfractionation. Pioneered by Cosden Oil & Chemical Company in 1957, it involves a series of three distillation columns, each with over 100 stages.
Several superfractionation units were constructed in the 1960s across the United States, Europe, and Japan. However, rising energy costs and high construction costs have made this method economically uncompetitive. As a result, superfractionation has largely been abandoned in favor of more efficient methods.
4. Uses of Ethylbenzene
Nearly all commercially produced ethylbenzene is used internally within refineries or chemical plants for the production of styrene monomer, which is a crucial building block for the production of polystyrene. In some cases, ethylbenzene is co-produced with propylene oxide.
Less than 1% of ethylbenzene is employed as a paint solvent or an intermediate for the synthesis of diethylbenzene, acetophenone, and ethylanthraquinone.
5. Toxicology of Ethylbenzene
Ethylbenzene is readily absorbed following inhalation, oral, and dermal exposure. It undergoes primary metabolism through side-chain oxidation, with minor metabolites from aromatic-ring hydroxylation. Less than 5% is excreted unchanged in urine as 2- and 4-ethylphenol.
The major identified metabolites are:
- 1-Phenylethanol (major urinary metabolite in rats)
- Mandelic acid (major urinary metabolite in humans)
- Phenylglyoxylic acid (major urinary metabolite in humans)
- Benzoic acid
- Phenylacetic acid (minor metabolite)
Experimental studies indicate low acute toxicity in animals. Oral LD50 values in rats range from 3.5 to 4.7 g/kg body weight. A dermal LD50 value of approximately 15 g/kg body weight has been reported.
Inhalation exposure in rats resulted in respiratory tract irritation starting at 1000 ppm. Higher concentrations caused unsteadiness, staggering gait, and ultimately unconsciousness and death at 5000 ppm. Human volunteers exposed to 25 ppm for 7.5 hours reported mild mucous membrane irritation, which became more pronounced at 100 ppm.
Repeated dermal contact with undiluted ethylbenzene can lead to erythema, edema, and superficial necrosis. Similar effects are anticipated in humans due to defatting of the skin with repeated exposures.
Studies examining repeated-dose exposure revealed that significant adverse effects in animals only occurred at relatively high doses of ethylbenzene.
No effects were observed in rats at 100 ppm and mice at 500 ppm over a three-month inhalation study; however, increased lung, kidney, and liver weights were observed starting at 250 ppm in rats and 750 ppm in mice. No histopathological changes were noted.
Two-year inhalation studies showed no effects in rats and mice at 75 ppm. Slight changes were observed at 250 ppm, with increased hepatic and pulmonary tumors in mice and renal tumors in male rats at 750 ppm (considered rat-specific and not relevant to humans).
Concerning ethylbenzene reproductive toxicity, conflicting results have been reported across various studies. Some studies suggest potential fetotoxicity at high exposure levels in pregnant mice and rats, but no clear effects were observed in rabbits at 1000 ppm with a 7-hour daily exposure.
The overall result of different studies suggests that ethylbenzene is not a mutagenic hazard. Limited in vitro data available for most metabolites also suggests no clear genotoxic potential.
A two-year inhalation study showed increased tumor incidence in male rats (kidney), male mice (lung), and female mice (liver) at the highest exposure level (750 ppm). No exposure-related tumors were observed in an oral study with rats and mice exposed to xylene mixtures containing 17% ethylbenzene.
Ethylbenzene is not genotoxic in vivo, suggesting a non-genotoxic mode of action for the observed tumors in the high-dose inhalation study. Increased tumor formation may be linked to ethylbenzene-induced cell proliferation in the kidney (rats) and lung/liver (mice).
Further investigation is needed to determine its relevance to humans and establish a safe occupational exposure limit.
IARC classifies ethylbenzene as “possibly carcinogenic to humans” (Group 2B) due to sufficient evidence in animals and inadequate evidence in humans.
The German MAK Commission classifies ethylbenzene as a category 3A carcinogen due to carcinogenicity data. They are awaiting further mechanistic data before establishing an occupational exposure limit.
ACGIH classifies ethylbenzene as an A3 carcinogen (“confirmed animal carcinogen with unknown relevance to humans”) and recommends a TLV-TWA of 100 ppm and a TLV-STEL of 125 ppm. They also define a biological exposure index (BEI) for mandelic acid in urine of 1.5 g/g creatinine.
In the EU, ethylbenzene is classified as “highly flammable” (F) and “harmful by inhalation” (Xn).
Reference
- Ethylbenzene; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a10_035.pub2
