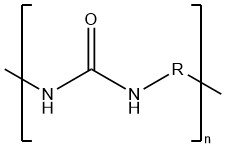
Polyureas refer to polymers with ureylene groups–NHCONH–in their polymer chain. Linear polyureas are thermoplastic polycondensation products, featuring either aromatic (R = arylene) or aliphatic (R = alkylene) structures.
Aliphatic polyureas display a difference of 50–100 °C between the onset of decomposition and the softening point. They are suitable for use as moldings.
Aromatic polyureas possess softening points that are close to their decomposition temperatures and are soluble in organic solvents, particularly in aprotic dipolar solvents like DMF, N-methylpyrrolidinone (NMP), and DMSO.
They are employed in the production of lacquers, varnishes, and coatings.
Table of Contents
1. Production of polyureas
The production of polyureas involves reactions between polyamines, predominantly diamines, featuring aliphatic, aromatic, or heterocyclic structures, and various substances such as carbon dioxide, carbonyl sulfide, carbonic esters, phosgene, urea, urethane, and isocyanates.
The only reactions that are technically significant include those between polyamines and polyisocyanates, as well as between isocyanates and water.
1.1. Production of polyureas from Diisocyanates and Diamines
Polyureas are directly produced by a polyaddition reaction between diisocyanates and diamines.
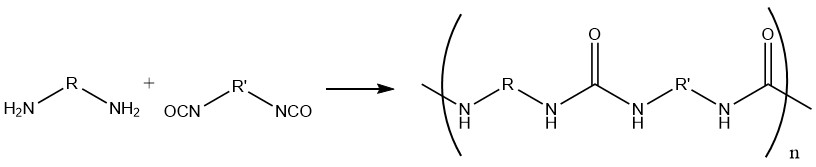
Isocyanate groups attached to an aromatic ring exhibit greater reactivity compared to those attached to an aliphatic chain.
Numerous aliphatic amines display high reactivity towards isocyanates, even at low temperatures. Secondary aliphatic and primary aromatic amines demonstrate similar reactivity, whereas secondary aromatic amines exhibit lower reactivity.
In the reaction of amine-isocyanates, a catalyst such as an amine, urea, or carboxylic acid, which functions as a proton acceptor/donor, is employed.
The reactions between isocyanates and amines are generally autocatalytic, but they can be catalyzed by various other catalysts, amines, or acids. They are usually conducted through interfacial polyaddition or in solution.
1.2. Production of polyureas from Diisocyanates and Water
This reaction is used in the fabrication of foams, wherein the produced carbon dioxide serves as a foaming agent.
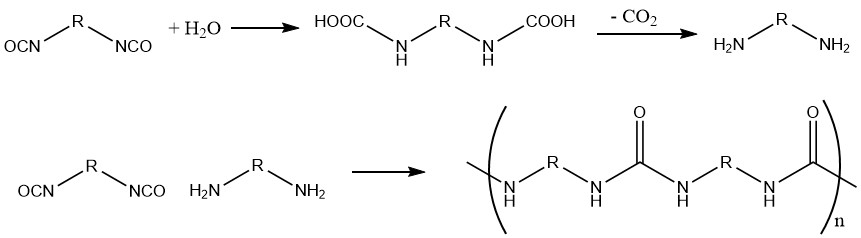
Different amines or amine complexes of metal salts can be used as catalysts.
2. Chemical properties of polyureas
Thermal Stability
Polyureas possess greater thermal stability than polyurethanes but lower thermal stability compared to polyamides. The thermal stability of the urea group is a multifaceted matter.
For instance, polyhexamethyleneurea (mp = 300°C) can be heated to 260°C without undergoing decomposition, while polydecamethyleneurea (mp = 230°C) decomposes quickly at 260°C in the molten state.
The mechanism underlying this thermal decomposition involves hydrogen transfer leading to the production of isocyanates and amines.
Hydrolytic Stability
Polyureas possess excellent stability towards hydrolysis under acidic and alkaline conditions.
3. Uses of polyureas
Polyureas have a wide range of applications, including their use as foams.
The production of polyureas by Reaction Injection Molding (RIM) is a well-established process that offers several advantages for high-volume production of large parts.
These advantages include low pressure (< 343 kPa), low temperatures (54–60°C), and the use of reactive liquid intermediates.
By formulating different polymers with polyols, isocyanates, and extenders, a wide range of properties can be achieved, from flexible elastomers to rigid plastics. The final product can be solid, microcellular, or foam-modified with various fillers.
Glass fiber reinforced RIM (RRIM) provides dimensional stability and high impact strength.
To reduce the weight of automobiles, high-modulus RIM and RRIM materials suitable for external body panels have been developed from polyurea elastomers, which are corrosion and impact resistant.
Advances in RIM technology include the development of new polyurea systems for the production of automobile body panels.
In automobile body panels, polyurea RIM materials offer greater impact resistance at equivalent glass-flake reinforcement, improvement in thermal stability for less distortion during paint cure cycles, and lower water absorption for better environmental resistance.
Polyurea RIM elastomers have several advantages, including the faster autocatalytic reaction of isocyanate with amine, resulting in high modulus polymers for automotive applications, the elimination of the need for catalysts, and the possibility of automation. The final product does not decompose or yellow, and their higher thermostability extends their practical utility.
Coating materials can also be made from polyureas in solution or emulsion.
Polyureas applied by spraying show excellent adhesion to a variety of substrates, including sand-blasted or primed steel, aluminum, and concrete.
The products include two-component sprayed polyurea elastomers, prepared from amine-terminated polyether resins and polyisocyanates. The polyurea spray elastomers systems require no catalyst and have extremely high reactivity and cure.
In other applications, aqueous polyurea dispersions can be used for coatings with improved hardness and solvent resistance.
Reference
- Polyureas; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.d21_d01.pub2