Sulfamic Acid: Properties, Reactions, Production and Uses
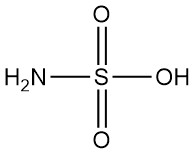
What is Sulfamic Acid?
Sulfamic acid, also known as amidosulfuric acid, is a strong inorganic acid with the chemical formula H3NSO3. It is a white, odorless, crystalline, and nonhygroscopic solid that is strongly dissociated in water.
Sulfamic acid has been manufactured industrially for approximately half a century. During the period from 1950 to 1980, production facilities were established in multiple industrialized nations in anticipation of increased market demand.
However, subsequent declines in product demand, along with production challenges and difficulties related to byproduct disposal, led to the closure of all manufacturing plants in Europe and the United States. Production continues in Japan (Nissan) and in Taiwan (multiple manufacturers).
Table of Contents
1. Physical Properties of Sulfamic Acid
Sulfamic acid is a white, crystalline, and non-volatile solid at ambient temperature. It forms a strong acid aqueous solution with a pH of 1.18 for only 1% solution. Sulfamic acid solubility in water is increased by adding other acids or salts and shows limited solubility in concentrated inorganic acids and most organic solvents.
Water solubility is dependent on temperature, as detailed in Table 1.
| Temperature (°C) | Solubility (g/100 g) | Concentration (wt %) |
|---|---|---|
| 0 | 14.7 | 12.8 |
| 10 | 18.5 | 15.6 |
| 20 | 21.3 | 17.5 |
| 30 | 26.1 | 20.7 |
| 40 | 29.5 | 22.8 |
| 50 | 32.8 | 24.7 |
| 60 | 37.1 | 27.0 |
| 70 | 41.9 | 29.5 |
| 80 | 47.0 | 32.0 |
Important physical properties of sulfamic acid are given in the following table.
| Property | Value |
|---|---|
| CAS Number | [5329-14-6] |
| Chemical Formula | H3NSO3 |
| Molecular Weight | 97.1 g/mol |
| Melting Point | 205 °C |
| Dissociation Constant | 1.10 x 10-1 |
| Density at 25 °C | 2.126 g/cm3 |
| Specific Heat | 1.1467 J/g |
| Vapor Pressure at 20 °C | 0.8 Pa |
| Vapor Pressure at 100 °C | 0.25 Pa |
2. Chemical Reactions of Sulfamic Acid
By heating sulfamic acid above 209 °C, it decomposes into sulfur trioxide, sulfur dioxide, water, ammonia, and nitrogen.
Aqueous sulfamic acid solutions hydrolyze to form ammonium hydrogen sulfate:
NH2SO3H + H2O → NH4HSO4
The hydrolysis rate is dependent on concentration, pH, and temperature. Dilute aqueous solutions are stable at ambient temperature. A 10% solution of sulfamic acid is hydrolyzed to 50% at a temperature of 80 °C for 10 hours.
Sulfamic acid can be oxidized in the presence of chlorine, bromine, and chlorates to produce sulfuric acid.
2 NH2SO3H + KClO3 → 2 H2SO4 + N2 + KCl + H2O
By heating concentrated nitric acid with sulfamic acid, dinitrogen oxide is formed.
NH2SO3H + HNO3 → H2SO4 + N2O + H2O
Nitrous acid reacts completely with sulfamic acid to yield nitrogen and sulfuric acid, so this reaction can be used for quantitative analysis.
NH2SO3H + HNO2 → H2SO4 + H2O + N2
Reaction with thionyl chloride results in the formation of sulfamyl chloride:
NH2SO3H + SOCl2 → ClSO2NH2 + SO2 + HCl
Sulfamic acid dissolves metal hydroxides, oxides, and carbonates.
Primary and secondary alcohols react with sulfamic acid to form alkylammonium sulfates.
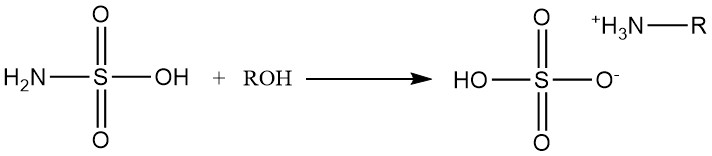
Secondary alcohol reactions necessitate the presence of amine catalysts, and tertiary alcohols do not react with sulfamic acid.
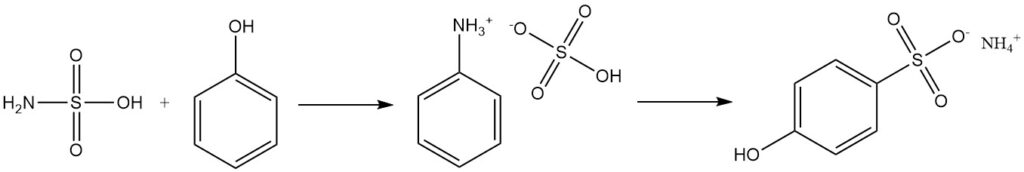
Aromatic alcohols like phenol react with sulfamic acid to produce sulfonates, with phenylammonium sulfate as an intermediate.
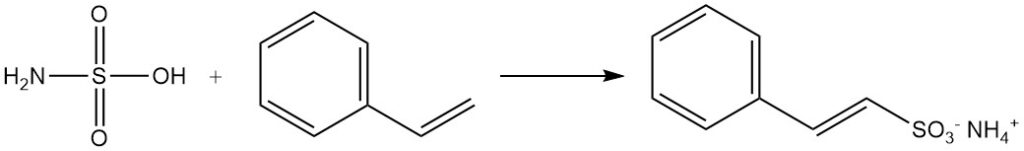
Aldehydes form addition products with sulfamic acid salts. Aromatic compounds with unsaturated side chains, such as styrene, undergo sulfonation by sulfamic acid while preserving the side chain double bond.
3. Production of Sulfamic Acid
Sulfamic acid production exclusively uses the urea process, with plants utilizing ammonia and sulfur trioxide having ceased operations.
Sulfamic acid is produced directly by reacting equimolar quantities of urea, sulfur trioxide, and sulfuric acid.
NH2CONH2 + SO3 + H2SO4 → 2 NH2SO3H + CO2
This reaction is strongly exothermic and involves a two-stage process, according to the following reactions:
NH2CONH2 + SO3 → NH2CONHSO3H
NH2CONHSO3H + H2SO4 → 2 NH2SO3H + CO2
In the initial stage, urea is stirred with excess sulfuric acid and sulfur trioxide. The temperature is maintained below 40 °C to inhibit carbon dioxide formation. The second stage involves reacting the product from the first stage in the presence of excess sulfur trioxide at a temperature range of 50–80 °C to yield sulfamic acid and carbon dioxide.
After the removal of excess sulfur trioxide, sulfamic acid with a purity exceeding 95% is obtained, and a high-purity product is obtained by recrystallization.
According to a Nissan patent, sulfamic acid is produced by reacting urea with oleum (xH2SO4 · ySO3).
The reaction product is wet recrystallized, followed by separation and drying of the crystallized acid. High-pressure grinding with a pressure ranging from 600–1500 kg/m2 results in a fine crystalline product with an average particle diameter of ≤ 500 μm.
4. Uses of Sulfamic Acid
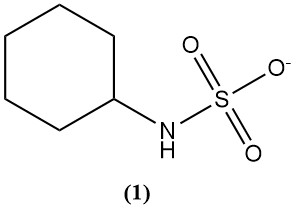
Sulfamic acid is primarily used to synthesize cyclamate (1) which is an artificial sweetener.
Sulfamic acid is also used in cleaning products to remove carbonate and phosphate deposits, such as boiler scale, because of its ability to form soluble salts and its relatively low corrosivity towards metals.
It is used in cleaning machinery and instruments across industries like paper, sugar, dairy, and brewing, as well as in eliminating deposits within evaporation plants, heat exchangers, and cooling systems.
Certain countries use sulfamic acid to treat fatty or ethoxylated alcohols in an industrial-scale process to produce wax precursors.
Sulfamic acid is also used as a chlorine stabilizer, in dye production, in electroplating processes, and in the paper and textile industries.
In laboratories, it is employed in analytical chemistry.
5. Toxicology of Sulfamic Acid
Sulfamic acid is generally considered to have low toxicity. Its physiological properties are characteristic of a strong mineral acid.
Sulfamic acid dust can irritate the mucous membranes of the nose and pharynx, as well as the conjunctiva of the eyes. This is a common effect of many strong acids.
Oral administration of 1.6 g/kg of sulfamic acid to rats has been found to be lethal. This indicates a certain level of toxicity, but it’s essential to consider that this is a relatively high dose and that the study was conducted on rats. Human toxicity may differ.
Sulfamic acid, like other strong acids, is corrosive to the skin, eyes, and respiratory tract. Direct contact should be avoided.
Proper handling procedures, including the use of personal protective equipment (PPE) such as gloves, goggles, and respiratory protection, are essential when working with sulfamic acid.
Reference
- Sulfamic Acid; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a25_439
