Industrial Production of Benzene
Benzene was traditionally produced from coal, but petroleum became the primary source in the mid-20th century due to new processes and increased demand. Catalytic cracking and reforming of petroleum fractions produce benzene and other aromatics.
Table of Contents
1. Production of Benzene from Coal
Coal carbonization produces light oils that contain benzene, toluene, and xylenes (BTX). The yield and composition of these oils depend on the type of coal and the carbonization temperature. Lower-temperature coking produces a clean-burning coke with a lower benzene content. High-temperature coking produces a hard coke for blast furnaces and light oils with a higher benzene content.
The light oils are separated from the coal tars and the BTX fraction is extracted using a high-boiling petroleum fraction. The BTX is then recovered from the absorbent oil and purified by distillation, sulfuric acid treatment, and hydrotreating. Solvent extraction and close fractionation are used to isolate individual aromatic components.
Various processes have been developed to refine the BTX cut into high-purity benzene or superior-quality BTX components. The Houdry-Litol process is an example of a process that uses a series of reactions and a catalytic reactor to refine the BTX cut. The modified Litol process is particularly adaptable for intricate coke-oven aromatic materials.
A small quantity of benzene can also be obtained from the distillation of coke oven tars, but this source is not of major significance in benzene production. Coal tars are more important as sources of pitches, naphthalene, anthracene, and other condensed aromatic materials.
2. Production of Benzene from Petroleum
Catalytic cracking and reforming of petroleum, developed in the late 1930s and 1940s, became a new source of benzene, toluene, xylenes, and higher aromatics. Petroleum replaced coal as the source of BTX materials, especially in the United States. The transition from coal to petroleum occurred in the United Kingdom and Europe around 1960.
Toluene was in high demand during World War II for the production of TNT. However, it was expensive to produce from virgin naphthas and later from catalytically cracked and reformed naphthas. One of the early catalytic cracking processes was developed by Houdry Corp.
This process used a silica-alumina catalyst deposited in several fixed beds and regenerated frequently with air to burn deposited coke. Another process developed by Mobil Oil used a moving-bed silica-alumina catalyst. The third type of process, developed by Exxon and others, is known as the fluid catalytic cracking (FCC) process and is now used exclusively.
Although the catalytic cracking processes were developed primarily to increase the yield and octane rating of motor fuels, the gasoline produced had a high aromatic content, providing a source of petrochemical benzene.
2.1. Benzene from Reformate
Catalytic reforming became another source of BTX aromatics from petroleum. It was installed in the 1940s and early 1950s to meet the growing demand for benzene, which was used to make resins, detergents, synthetic fibers, and other chemicals.
Reforming involves dehydrogenating naphthenes to aromatics, or isomerizing alkylnaphthenes followed by dehydrogenation. Paraffins can also be dehydrocyclized to aromatics. The reaction is slow, so process conditions must be adjusted to make it economical. A small amount of dealkylation of alkylated aromatics may also occur.
Early catalytic reforming units used base-metal catalysts, such as molybdena on activated alumina. These processes had a short cycle length and required frequent catalyst regeneration. The hydroforming process operated at elevated temperatures and pressures with hydrogen recycle. It converted naphtha fractions into high-octane gasolines. The catalyst required regeneration every 8-16 hours.
Later catalytic reforming processes used either moving catalyst beds or fluidized beds with a finely divided catalyst. Various catalytic processes using platinum on alumina and platinum with modifiers on alumina have also been developed.
These processes can have cycle lengths as long as six months or much shorter, depending on the feedstock, process conditions, and severity of operation. The naphtha feedstock must be pretreated with catalytic hydrotreating to remove sulfur compounds.
Commercial catalytic reforming processes include: catalytic reforming (Institut Français du Pétrole), magnaforming (Engelhard Industries), platforming (UOP), powerforming (Exxon Research & Engineering), rheniforming (Chevron Research Co.), and ultraforming (Standard Oil of Indiana).
Operating conditions vary widely. Temperatures range from 425 to 525 °C and gauge pressures from 0.7 to 3.5 MPa.
Naphthene conversions approach 100%. Cyclization of paraffins is much lower. For example, to produce benzene from C6 and higher stocks, methylcyclopentane is isomerized to cyclohexane and dehydrogenated to benzene. Conversion of n-hexane to benzene is limited.
Catalytic reformer units may be operated at relatively high pressures with a series of fixed-bed reactors. The catalyst is regenerated at defined intervals. Units designed for higher conversion operate at lower pressure with more frequent catalyst regeneration. Shorter cycles, swing reactors, or continuous regeneration are used.
The platforming process shown in Figure 1 employs continuous catalyst regeneration.
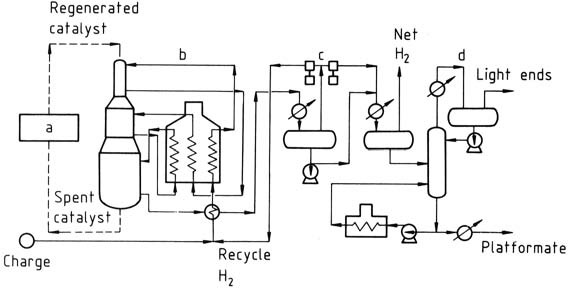
a) Catalyst regeneration; b) Reactors; c) Product separation; d) Stabilizer
Another design developed by Standard Oil of Indiana uses a swing reactor in which the fixed-bed catalyst in one reactor is regenerated and brought back onstream, with the catalysts in each of the remaining reactors being regenerated in rotation. Gauge pressures of 0.7-2.4 MPa are employed in the different processes.
For gasoline production, naphthas with boiling ranges of around 80-210 °C are used. If reforming is carried out for the production of aromatic charge stocks, the composition and boiling range of the naphtha may be altered.
The reformate may have a RON clear octane rating as high as 100 and may contain up to 70 vol% aromatics.
Over the years, the process has been made more effective. Reforming catalysts have been modified. Small amounts of water were found to enhance benzene yields with certain catalysts when producing BTX. Higher xylene yields have resulted from operating at increased space velocities.
2.2. Benzene from Pyrolysis Gasoline
Pyrolysis gasoline, a byproduct of ethylene production, is another good source of BTX. The amount of pyrolysis gasoline produced depends on the feedstock and operating conditions. It is higher with heavier feedstocks and can be up to 20% for medium-range naphtha.
The composition of pyrolysis gasoline varies with the boiling point of the feed, with heavier feeds producing more toluene and xylene than benzene.
Pyrolysis gasoline contains > 60% BTX, but it also contains unsaturates and diolefins due to the high temperature needed for ethylene production. The diolefin content is typically 3-5%, and the total content of cyclic olefins and cyclic diolefins is also 3-5%.
Pyrolysis gasoline is unstable because of the diolefinic material. Lower boiling diolefins can be removed by distillation, but this can be difficult due to their tendency to polymerize and depolymerize. Therefore, a two-stage hydrotreating process is generally used.
The first stage converts diolefins to olefins. If the gasoline is to be used as a fuel, further treatment may not be necessary. However, if the gasoline is a source of aromatics, a second stage is used to saturate olefins and remove residual sulfur.
3. Other Production Methods of Benzene
To meet demand, benzene production can be increased from other aromatic compounds, such as the BTX fractions obtained from catalytic reformate, pyrolysis gasoline, or light oils from coal carbonization. This can be done using hydrodealkylation, disproportionation, or combination processes.
3.1. Production of Benzene by Hydrodealkylation
Hydrodealkylation is a process of removing alkyl groups from aromatic compounds using hydrogen. It is a source of benzene, and toluene is the usual charge stock. Higher alkylated aromatics can also be used, but the reaction proceeds stepwise. Other alkylbenzenes, such as ethylbenzene and propylbenzene, dealkylate in a single step and form the corresponding alkanes.
Catalytic and thermal processes
Both catalytic and thermal processes are used for hydrodealkylation. Catalytic processes, such as Detol and Hydeal, operate at 575-650 °C and 2.5-6 MPa. Thermal processes, such as HDA and THD, operate at higher temperatures than catalytic processes, but yields and reaction systems are similar in both types.
Detol process
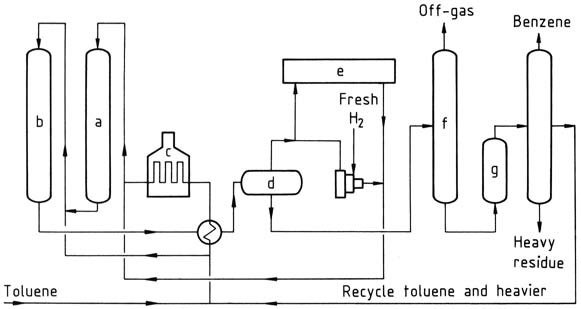
a) Reactor I; b) Reactor II; c) Furnace; d) Separator; e) Hydrogen purification; f) Stabilizer; g) Clay treater
The Detol process is a catalytic dealkylation process that uses a fixed-bed dealkylation catalyst. The feed consists of toluene or mixtures of toluene and other alkylated benzenes. The alkylated aromatics and hydrogen pass at elevated temperature and pressure over the catalyst in series.
Heat exchangers cool the reactor products and condense benzene, unreacted toluene, and the heavier unreacted alkylated benzenes. A high-pressure flash drum separates recycle hydrogen and product gas. The product gas is split into streams for fuel gas, for hydrogen purification if needed, and for recycle hydrogen.
The liquid from the flash drum is pumped to a stabilizer, where remaining gas and low-boiling hydrocarbons are driven off and utilized as fuel gas. The bottoms from the stabilizer are treated by clay and passed to a column where the benzene is distilled overhead.
Unreacted toluene and heavier aromatics are recycled. The benzene produced in yields of around 99 mol% is highly pure, typically 99.95 %, with a freezing point of 5.45 °C.
Hydeal process
The Hydeal process is another catalytic dealkylation process. It is similar to the Detol process, but uses a chromia-alumina catalyst instead. Nitration-grade benzene is obtained in yields of ca. 98 mol%. Unreacted aromatic charge material from the once-through operation is recycled to obtain practically complete conversion. The process is also used to hydrodealkylate alkylnaphthalenes.
HDA and THD processes
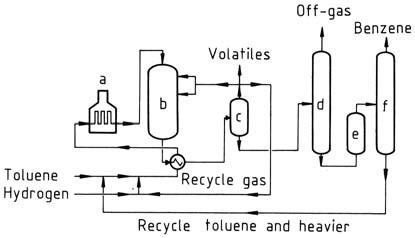
a) Heater; b) Reactor; c) Separator; d) Stabilizer; e) Clay treater; f) Distillation
The HDA and THD processes are thermal dealkylation processes. They operate at higher temperatures and pressures than catalytic processes. Benzene yields of 99 mol% are achieved.
MHC process
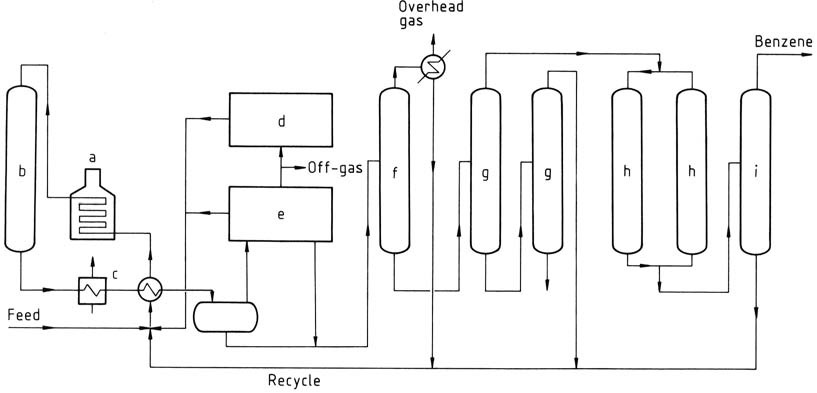
a) Heater; b) Reactor; c) Waste-heat boiler; d) Hydrogen regeneration; e) Aromatic recovery; f) Fractionation; g) Vacuum columns; h) Clay treaters; i) Benzene column
The MHC process is a thermal dealkylation process developed by Mitsubishi Petrochemical Co. It can handle feedstocks containing up to 30% of nonaromatics without resorting to aromatic extraction and fractional distillation steps. The process will operate on low-purity hydrogen, thus reducing the amount of makeup hydrogen required. Benzene of 99.95% purity is readily achieved.
3.2. Production of Benzene by Disproportionation
The Tatoray process is a way to make benzene and xylenes from toluene and C9 aromatics. It uses a noble-metal or rare-earth catalyst and recycles hydrogen to make the reaction work. The process operates at 350-525 °C and 1-5 MPa.
The benzene produced is very pure, and the xylenes contain very few saturated hydrocarbons. When the feedstock is limited to C9 and C10 aromatics, the process produces a mixture of benzene, toluene, and xylenes.
The Tatoray process is used in a number of commercial plants around the world. The yield of benzene and xylenes is about 97% when toluene is used as the feedstock.
3.3. Production of Benzene by Combination Dealkylation Processes
Several processes have been developed to produce benzene from impure BTX feedstocks by combining hydrogenation and dealkylation.
One example is the Pyrotol process, which involves fractionating the feed to remove light and heavy compounds, vaporizing the C6-C8 fraction, and passing it through a catalytic reactor to selectively hydrogenate diolefins, cyclic diolefins, and styrene.
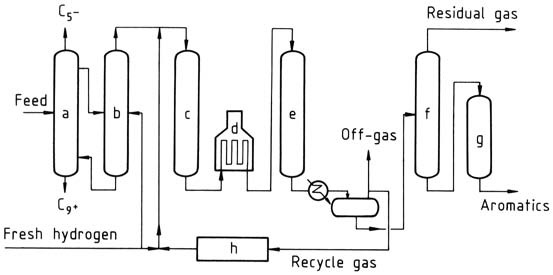
a) Distillation; b) Vaporizer; c) Prereactor; d) Heater; e) Pyrotol reactor; f) Stabilizer; g) Clay treater; h) Hydrogen purification
The effluent from the pretreat reactor is then charged to the Pyrotol reactors, where aromatics are dealkylated to benzene. Other reactions include desulfurization and hydrocracking of nonaromatics. Unreacted toluene and heavier aromatics are recycled. The benzene product contains less than 0.5 ppm of thiophene and has a freezing point of at least 5.47 °C.
Another example is the Litol process, developed by the Houdry Division of Air Products and by Bethlehem Steel. This process produces pure benzene from aromatic light oil obtained from coal carbonization. It utilizes two catalytic reaction stages.
After prefractionation of the raw light oil to remove C5 and lighter fractions overhead and C9 and heavier as bottoms, the C6-C8 fraction is vaporized. At the first stage, diolefins and styrene are hydrogenated.
The effluent then contacts the Litol chromia-alumina catalyst at 500-600 °C, where desulfurization, hydrocracking, and dealkylation occur. Benzene purity of 99.97% with freezing point of 5.5 °C and a thiophene content of less than 0.5 ppm are typical.
A process was developed by Mitsubishi Petrochemical Co. for producing benzene from pyrolysis gasoline. This process involves two-stage hydrogenation followed by solvent extraction to extract the aromatics, or alternatively, reaction of the hydrogenated material in Mitsubishi’s MHC process.
Mobil Chemical Co. has developed a liquid-phase disproportionation process for converting toluene to benzene and xylenes. This low-temperature disproportionation (LTD) process employs a highly active zeolite-based catalyst that allows the reaction to proceed at temperatures as low as 260 °C. Hydrogen recycle is not required in this liquid-phase process.
Reference
- Benzene; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a03_475
