Maleic Acid: Properties, Reactions, Production and Uses
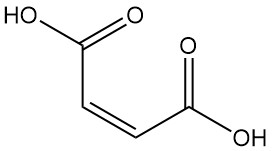
What is Maleic Acid?
Maleic acid, also known as cis-butenedioic acid, is a dicarboxylic acid with the formula C4H4O4. It appears as a colorless, crystalline solid with a faint odor.
Maleic acid is not found in nature and was first synthesized in 1834 by heating malic acid. Maleic acid became industrially important after commercial production began in 1919 via the catalytic gas-phase oxidation of benzene.
Maleic acid and its anhydride became commercially available in 1928 and 1933, respectively. Maleic acid has limited industrial applications on its own, but it is used as a precursor for maleinate resins and copolyesters.
Table of Contents
1. Physical Properties of Maleic Acid
Maleic acid forms monoclinic prism crystals when crystallized. It is very soluble in water and ethanol, soluble in acetone and glacial acetic acid, slightly soluble in ether, and insoluble in benzene.
Table 1 summarizes the physical properties of maleic acid.
| Property | Value |
|---|---|
| CAS number | [110-16-7] |
| Chemical formula | C4H4O4 |
| Molecular weight | 116.07 g/mol |
| Melting Point | 130.5 °C |
| Density | 1.590 g/cm3 |
| pKa1 at 25 °C | 1.94 |
| pKa2 at 25 °C | 6.23 |
| Heat of formation | -788.3 kJ/mol |
| Heat of combustion | -1358.9 kJ/mol |
| Solubility in water |
|
| Flash Point | 127 °C |
2. Reactions of Maleic Acid
Maleic acid exhibits high reactivity due to the presence of both carboxyl groups and a double bond. Above 100 °C, it is dehydrated by eliminating water to form maleic anhydride. Further heating, especially with catalysts, promotes decarboxylation to yield acrylic acid.
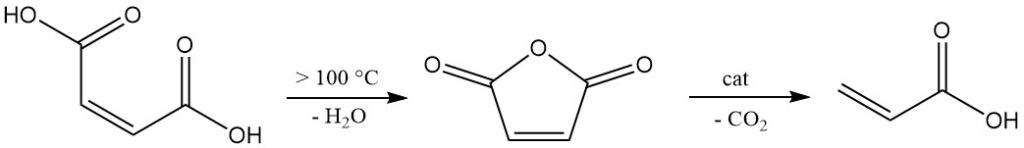
Maleic acid participates in typical carboxylic acid reactions like esterification and amidation, but it is incapable of forming acid chloride.
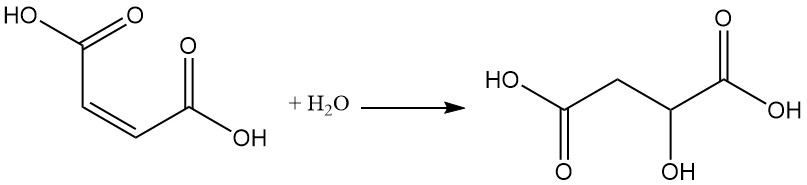
The presence of the double bond allows for various reactions, such as the addition of water at elevated temperature and pressure, which results in malic acid formation.
The reaction of maleic acid with ozone yields glyoxylic acid.
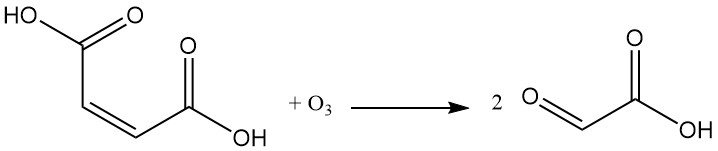
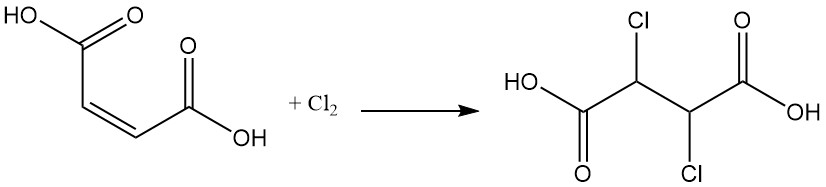
Halogen addition forms dihalogenosuccinic acid compounds; for example, the addition of dichlore to maleic acid produces dichlorosuccinic acid.
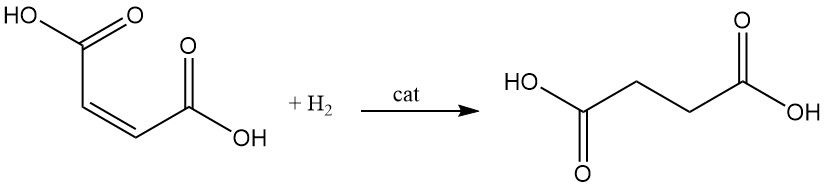
The catalytic hydrogenation of the double bond of maleic acid leads to the formation of succinic acid.
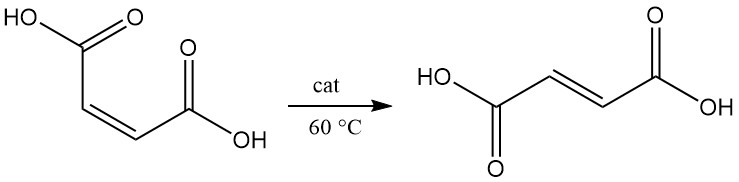
Isomerization of maleic acid to fumaric acid occurs slowly, even at 100 °C. In the presence of a catalyst, the conversion proceeds nearly quantitatively, even at 60 °C.
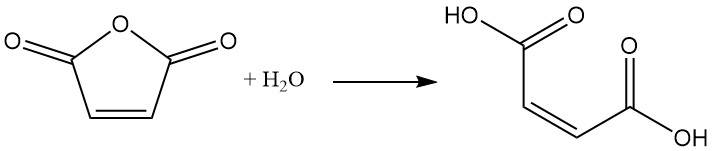
3. Production of Maleic Acid
The most common method for maleic acid production is the controlled hydration of maleic anhydride with a small quantity of water. After the reaction mixture is cooled, the product, maleic acid, precipitates as a solid.
The supernatant liquid (mother liquor) is then separated. The solid maleic acid is dried under vacuum.
The mother liquor can either be diluted and recycled back to the hydration stage for further maleic acid production or directed to the dehydration unit for maleic anhydride production, eliminating the need for separate effluent treatment. However, this method requires corrosion-resistant steel equipment due to the harsh conditions.
As an alternative, maleic acid can be obtained directly from the wash water generated during maleic anhydride production. This process saves the energy required for dehydration to the anhydride form. However, it necessitates the purification and concentration of the wash water.
The wash water must be treated with activated charcoal to remove impurities. The spent charcoal is then disposed of. The purified solution needs to be concentrated under vacuum because elevated temperatures promote fumaric acid formation, and the chrome-nickel steel equipment used in this process corrodes above 80 °C.
Enriched wash water streams from oxidation processes such as o-xylene and naphthalene used in phthalic anhydride production can also be used as a starting material for maleic acid production, alongside fumaric acid and maleic anhydride.
4. Uses of Maleic Acid

Maleic acid is an important component in polyester production used for fiber-reinforced laminated moldings and paint vehicles.
It is used as a starting material for the synthesis of numerous other chemicals.
Maleic acid can be converted to fumaric acid, another valuable industrial chemical.
Due to its acidic properties, it can be used as an acidity regulator in certain foods and beverages.
It enhances adhesion between various materials, such as zinc-coated metals (galvanized steel) and nylon, used with methyl methacrylate-based adhesives.
Maleic acid is applied in dyeing and finishing processes for natural fibers.
The ionized form (maleate ion) acts as an inhibitor for transaminase reactions in biochemical research.
Maleic acid is used as a buffering agent, foaming agent, and pH regulator in cleaning products and household care applications.
5. Toxicology of Maleic Acid
Maleic acid’s primary toxicological concern is its local irritant and corrosive effect on skin, mucous membranes, and eyes. Studies show that a 20% aqueous solution can cause mild and reversible skin irritation in humans, while lower concentrations (<5%) can significantly irritate the eyes.
Animal experiments confirm this local irritant action as the most prominent toxicological feature.
Maleic acid shows moderate acute oral and dermal toxicity. LD50 values reported are:
- 708 mg/kg (rat, oral)
- 2400 mg/kg (mouse, oral)
- 1560 mg/kg (rabbit, dermal)
- >1000 mg/kg (guinea pig, dermal)
Kidney damage is a potential systemic toxic effect of maleic acid. Morphological and functional changes in kidneys were observed in rats and dogs upon intraperitoneal injection and inhalation exposure.
This damage resembles Fanconi syndrome, potentially caused by the interaction of maleic acid with glutathione in kidney cells, leading to free radical and peroxide formation. Additionally, maleic acid seems to affect sodium and hydrogen ion transport mechanisms.
Chronic exposure studies in male rats (250–750 mg/kg/day for up to two years) showed increased mortality, kidney damage, and delayed growth in all dosage groups. Liver and testicular damage were also observed in the highest-dose group.
No carcinogenicity or genotoxicity was evident in these studies, although they were not specifically designed for this purpose.
References
- Maleic and Fumaric Acids; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a16_053
- https://pubchem.ncbi.nlm.nih.gov/compound/Maleic-Acid
