Carbon disulfide: properties, reactions, production and uses
Carbon disulfide is a colorless liquid with the formula CS2. It is a significant chemical compound widely employed in various industrial applications.
The discovery of carbon disulfide can be attributed to Lampadius in 1796, who discovered it while heating a combination of iron pyrite and carbon.
The compound garnered industrial interest as early as 1839, when Schrötter successfully synthesized it using charcoal and sulfur in a heated retort. Carbon disulfide’s remarkable solvent capabilities were quickly recognized, leading to its widespread use in the extraction of fats and oils for a considerable period.
However, its true significance as a large-scale industrial chemical emerged with the advent of the viscose rayon process in the early 1900s. This process relied on carbon disulfide to dissolve cellulose, thereby establishing its pivotal role in the industry.
Nonetheless, the rapid growth of rayon faced setbacks in the late 1960s due to competition from petroleum-based synthetic fibers, resulting in a subsequent decline in both the rayon and carbon disulfide markets throughout the 1970s. However, in recent times, the usage of carbon disulfide has stabilized, and there is projected small market growth for the future.
In 1984, out of the total worldwide production of 1.1 million tons of carbon disulfide, more than 75% was primarily consumed by the regenerated cellulose industry. The remaining portion found its application in the production of carbon tetrachloride and diverse organosulfur compounds, which are extensively used as rubber chemicals, flotation agents, and pesticides.
Table of Contents
1. Physical properties of carbon disulfide
Carbon disulfide is a highly volatile liquid of significant density, exhibiting a broad flammability range in the presence of air, spanning from 1 to 50 volume percent. It possesses an exceptionally low autoignition temperature of 100 °C and a flash point of -30 °C. Its rate of evaporation into the atmosphere surpasses that of diethyl ether by a factor of 1.6.
This compound serves as an exceptional solvent for numerous organic substances, readily dissolving sulfur, phosphorus, iodine, waxes, rubber, and resins. The commercially available form emits a pungent aroma, characteristic of sulfur-based compounds.
Some of the most useful physical properties of carbon disulfide are as follows:
- Molar mass = 76.13 g/mol
- Fusion point = -111.6 °C
- Boiling point = 46.23 °C
- Density at 25 °C = 1.26 g/ml
- Critical temperature = 273 °C
- Critical pressure = 7600 kPa
- Surface tension at 20 °C = 32.3 mN/m
- Refractive index at 20 °C = 1.62546
- Viscosity at 25 °C = 0.36 mPa.s
2. Chemical Reactions of carbon disulfide
Carbon disulfide exhibits a wide range of chemical reactions that have been extensively studied and documented.
When carbon disulfide reacts with sulfides, alcohols, ammonia, amines, and chlorine, it generates industrially significant derivatives and intermediates. In the presence of aqueous alkalies, carbon disulfide reacts slowly to form trithiocarbonate and carbonate compounds, as represented by the equation:
3 CS2 + 6 NaOH → 2 Na2CS3 + Na2CO3 + 3 H2O
Metal sulfides form trithiocarbonates when reacted with carbon disulfide, as shown in the reaction:
K2S (aqueous) + CS2 → K2CS3
Moreover, when carbon disulfide reacts with alcoholic alkalies, it yields xanthates, which are salts of dithiocarbonates, through the following process:
ROH + NaOH → RONa + H2O
RONa + CS2 → ROCSSNa
This reaction serves as the foundation for the viscose process, in which cellulose is converted into xanthate and subsequently regenerated as fibers and films, as demonstrated in the reaction:
(cellulose)ONa + CS2 → (cellulose)OCSSNa
2(cellulose)OCSSNa + H2SO4 → 2(cellulose)OH + 2 CS2 + Na2SO4
In the presence of ammonia, carbon disulfide forms ammonium dithiocarbamate (1), ammonium trithiocarbonate (2), and ammonium thiocyanate (3), depending on the concentration of ammonia and temperature:
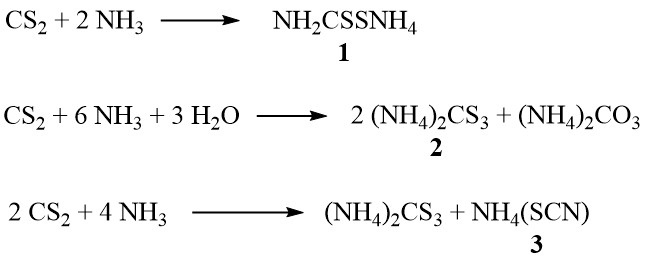
Additionally, at higher temperatures in the vapor phase, the thiocyanate decomposes to produce thiourea:
NH4(SCN) → SC(NH2)2
Primary and secondary amines predominantly yield dithiocarbamate salts in their reaction with carbon disulfide:
2 RNH2 + CS2 → RNHCSSNH3R
2 R2NH + CS2 → R2NCSSNH2R2
In the presence of alkalies, various commercially important salts of dithiocarbamic acid can be obtained:
R2NH+ CS2 + NaOH → R2NCSSNa + H2O
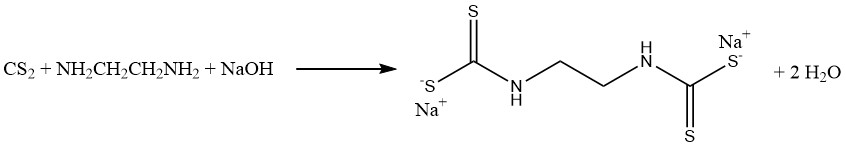
The reaction of carbon disulfide with methylamine and caustic soda in an aqueous solution produces sodium methyldithiocarbamate (CH3NHCSSNa), an essential soil fumigant. Similarly, carbon disulfide reacts with ethylenediamine to yield the fungicide sodium ethylene bis(dithiocarbamate):
Aniline and carbon disulfide reactions result in the formation of two significant vulcanization accelerators. In the absence of sulfur, thiocarbanilide is produced, while in the presence of sulfur, 2-mercaptobenzothiazole (MBT) is obtained:
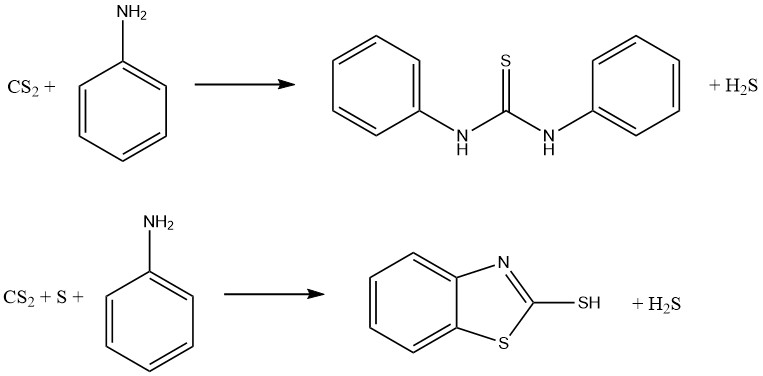
Dithiocarbamates can be oxidized to form a family of compounds known as thiurium disulfides, which are used as fungicides and vulcanization accelerators. The reaction involves the following equation:
2 R2NCSSNa + H2O2 + H2SO4 → (R2NCSS–)2 + Na2SO4 + 2 H2O
Chlorination of carbon disulfide leads to the formation of various chlorinated products. Under iodine catalysis, chlorination at temperatures between 5 and 30 °C produces trichloromethanesulfenyl chloride, along with carbon tetrachloride, sulfur dichloride, and thiophosgene, depending on the degree of chlorination.
In the presence of iron and metal chlorides at higher temperatures (70 – 100 °C), carbon tetrachloride and sulfur chlorides are exclusively obtained.
Carbon disulfide readily reacts with water in the presence of alumina and oxide catalysts above 150 °C, producing carbon dioxide and hydrogen sulfide. Carbonyl sulfide acts as an intermediate in this hydrolysis reaction. At temperatures ranging from 200 to 300 °C, the conversion of carbon disulfide to these products reaches equilibrium.
When subjected to high temperatures (above 150 – 200 °C) in the presence of metal sulfide catalysts like molybdenum and nickel sulfides, carbon disulfide undergoes reduction by hydrogen, resulting in varying amounts of methanethiol, dimethyl sulfide, methane, and hydrogen sulfide.
3. Production of carbon disulfide
The carbon disulfide is produced by the reaction of sulfur with charcoal or methane. Although ethane, propane, and propene have been used to a limited extent, the methane process has gained significant prominence since its introduction in the early 1950s.
Consequently, the older charcoal process is no longer relevant in the manufacturing of carbon disulfide in the United States, Europe, and Japan. However, in regions where natural gas or methane is not easily accessible or when plant size is relatively small, the charcoal process is still employed to meet local demands for viscose rayon production.
3.1. Production of carbon disulfide from Charcoal and Sulfur
The fundamental process for the reaction of charcoal with sulfur has undergone minimal changes since its inception in the mid-1840s. Externally heated retorts are still utilized, although equipment design has significantly improved with the availability of superior construction materials.
In retort plants, molten sulfur is vaporized and superheated either before or after being introduced into the retort. Within the retort, at temperatures ranging from 850 to 900 °C and slightly above atmospheric pressure, the superheated sulfur vapor reacts with a stationary bed of charcoal.
The resulting product gas, which includes carbon disulfide, sulfur, hydrogen sulfide, carbonyl sulfide, and inert gases, passes through a series of condensers, scrubbers, and oil absorbers to recover crude carbon disulfide.
Further treatment in distillation columns yields the pure product. The tail gas, consisting primarily of hydrogen sulfide, carbonyl sulfide, and inert gases, can either be incinerated and scrubbed with a caustic solution or directed to a sulfur recovery plant.
Typically, each retort is capable of producing 1 to 3 metric tons per day of carbon disulfide. The useful life of cast iron retorts is limited to less than one year due to high-temperature corrosion. To minimize the need for reactor cleanouts, the raw materials used in the retorts should have low ash and residue content. Fouling of the retorts hampers heat transfer and reduces their lifespan.
The quality of charcoal is crucial, as less reactive materials require higher temperatures for satisfactory operation. Charcoal derived from hardwood is preferred for this reason.
The charcoal is typically precalcined at 500 °C to remove volatile substances that could lead to the formation of undesirable byproducts. The thermal efficiency of retorts is approximately 25%, with carbon and sulfur yields generally below 90%.
An alternative heat source for the reaction of charcoal with sulfur is provided by electrical resistance heating in brick-lined electrothermal reactors. When inexpensive power is available, the electrical method may be more cost-effective.
The power consumption per metric ton of carbon disulfide produced is around 1200 kWh. Electrothermal reactors offer several advantages, including larger capacity per reactor (up to 10 metric tons per day) and an extended reactor lifespan. Additionally, a wider range of solid carbon feed can be utilized as electrothermal reactors can generate higher temperatures.
3.2. Production ofcarbon disulfide from Methane and Sulfur
Due to the growing demand for carbon disulfide in the 1940s, the limitations of charcoal processes prompted the development of a more efficient large-scale manufacturing method.
The catalyzed reaction between methane and sulfur, leveraging readily available and cost-effective natural gas, emerged as a successful alternative. Numerous patents were issued between 1943 and 1965, covering various aspects of this process.
The commercial methane process, primarily based on the patents acquired by the Pure Oil Co., was later taken over by the Food Machinery Corp. (FMC) and Stauffer Chemical Co.
In 1953, FMC built the first methane process plant in South Charleston, West Virginia, followed by a Stauffer plant in LeMoyne, Alabama, three years later. By the mid-1960s, all carbon disulfide production in the United States was accomplished through this method, and similar plants soon emerged in Europe and other regions.
Currently, over 85% of the world’s production capacity for carbon disulfide is based on the methane process.
A typical methane process flow sheet is illustrated in Figure 1. Purified natural gas, containing over 95% methane and low concentrations of propane and heavier hydrocarbons, is preheated to approximately 250 °C in the convection section of the reaction furnace.
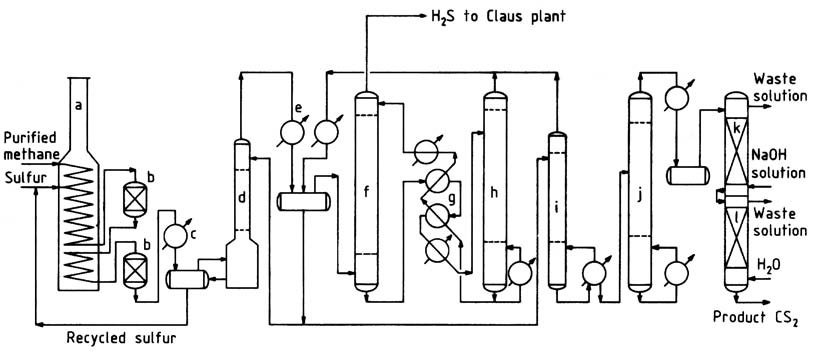
a) Reaction furnace; b) Catalytic reactors; c) Sulfur condenser; d) CS2 scrubber; e) CS2 condenser; f) CS2 absorber; g) Oil interchangers; h) CS2 stripper; i) Stabilizer column; j) Purification column; k) Caustic wash column; l) Water wash column
The furnace, a box-type pyrolysis furnace commonly employed in the petrochemical industry, consists of radiant and convection sections. The hot methane gas is mixed with liquid sulfur and introduced into the horizontal heating coil located in the radiant section.
In this section, the liquid sulfur vaporizes, and the temperature of the mixture rises to 550–650 °C. The operating pressure typically ranges from 400 to 700 kPa.
Alternatively, methane and sulfur can be introduced separately and at different locations within the coil. A slight excess of sulfur (around 5%) is employed to ensure efficient methane conversion and minimize carbon and tar formation.
To withstand the corrosive high-temperature environment, cast high-alloy stainless steel is used as tube material, providing a tube life of approximately 2–3 years. Packed, brick-lined reactors are connected to the heating coil to offer additional residence time and housing for catalyst beds.
The reaction takes place in both the tubes and reactors, ensuring almost complete conversion of hydrocarbons before the gas exits the last reactor.The gas leaving the reaction furnace system passes through a sulfur condenser, where most of the excess sulfur is separated.
Subsequently, the cooled gas proceeds to a sulfur scrubber and then to the CS2 scrubber, where the remaining sulfur is removed. The relatively sulfur-free gas enters water-cooled condensers for bulk separation of carbon disulfide.
The remaining carbon disulfide is extracted from the hydrogen sulfide gas in the oil absorber. The hydrogen sulfide gas leaving the oil absorber contains approximately 1-2% methane and less than 1000 ppm carbon disulfide.
This gas is directed to a Claus sulfur recovery plant, where it undergoes conversion back to sulfur.
Crude carbon disulfide, including that recovered in the stripper, is sent to the stabilizer column to eliminate low-boiling impurities.
The bottoms from the stabilizer column then undergo processing in the purification column to separate high-boiling contaminants from the product. Product purification is completed through caustic and water washes, after which the finished product is pumped to check tanks and storage.
4. Uses of carbon disulfide
The primary consumer of carbon disulfide is the regenerated cellulose industry, which accounts for over 65% of world production. Specifically, the manufacture of rayon utilizes a significant portion of carbon disulfide, with approximately 0.32 kg of carbon disulfide consumed for each kilogram of rayon produced.
Around 10% of carbon disulfide is utilized in the production of cellophane, while another 10% is chlorinated to produce carbon tetrachloride. This method accounts for approximately 25% of the global carbon tetrachloride production.
The remaining 15% of carbon disulfide is distributed among various applications, including rubber chemicals, flotation agents, pesticides, miscellaneous chemical reagents, extraction solvent, catalyst presulfidation agent and oil well solvent.
However, the regenerated cellulose industry faces challenges due to competition from synthetic fibers and films, which limits its market growth. To foster progress, there is a need for gradual improvement in carbon disulfide usage, particularly through increased utilization in the manufacturing of agricultural and specialty chemicals.
Reference
- Carbon Disulfide; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a05_185
