Acetamide: Properties, Production and Applications

What is acetamide?
Acetamide, also known as ethanamide or acetic acid amide, is an organic compound with the chemical formula C2H5NO. It is a white, odorless, hygroscopic solid formed from acetic acid and ammonia. Acetamide has been naturally found in the roots of sugar beets and in coal mine waste dumps as a minor by-product of organic material degradation.
Table of Contents
1. Physical properties of acetamide
Pure acetamide has a bitter taste and is odorless. A characteristic mousy odor is attributed to trace impurities, possibly from acetonitrile. Acetamide occurs in two crystalline forms: a stable trigonal form and a metastable orthorhombic modification. It is very soluble in water, ethanol, and chloroform, glycerin, hot benzene and slightly soluble in diethyl ether.
Table 1 lists the important physical properties of acetamide.
| Property | Value |
|---|---|
| CAS Registry Number | 60-35-5 |
| Molecular formula | C2H5NO |
| Molar mass | 59.07 g·mol−1 |
| Melting point | 81–82 °C |
| Melting point (trigonal) | 80.0–80.1 °C |
| Triple point | 353.33 K |
| Boiling point (1 atm) | ≈221 °C |
| Density (20 °C) | ≈1.16 g/cm3 |
| Melt density at 85 °C | 0.9986 g·mL−1 |
| Heat of melting (ΔHm) | 264 kJ·kg−1 |
| Dielectric constant | 59 |
| Dipole moment | 12.41 × 10−30 C·m |
| Vapor pressure at 272 K | 10 kPa |
| Vapor pressure at 278 K | 20 kPa |
| Vapor pressure at 281 K | 30 kPa |
| Vapor pressure at 284 K | 40 kPa |
| Vapor pressure at 285 K | 50 kPa |
| Vapor pressure at 287 K | 60 kPa |
| Vapor pressure at 288 K | 70 kPa |
| Vapor pressure at 290 K | 80 kPa |
| Vapor pressure at 291 K | 90 kPa |
| Vapor pressure at 292 K | 100 kPa |
2. Chemical properties of acetamide
Acetamide is a simple aliphatic amide and exhibits properties characteristic of the amide functional group.
Acetamide shows amphoteric behavior when dissolved in water. Hydrolysis in neutral water occurs only slowly, but in the presence of strong acids or bases, it reacts instantly to produce acetic acid and ammonia.
The autodissociation constant of acetamide is about 3.2 × 10-11 at 94 °C, which demonstrates a low tendency for self-ionization under thermal conditions.
Acetamide reacts with mineral acids to form solid complexes. Salts of hydrogen bromide, hydrogen chloride, and nitric acid with acetamide have been isolated and characterized.
The molten phase of acetamide is used to dissolve metal salts that have been evaluated for conductivity, stability, and suitability for electrodeposition.
3. Production of Acetamide
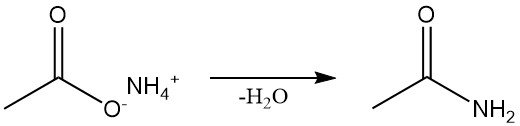
Acetamide is obtained by several synthetic routes. It is produced industrially by a continuous dehydration of ammonium acetate, which is also the standard laboratory method.
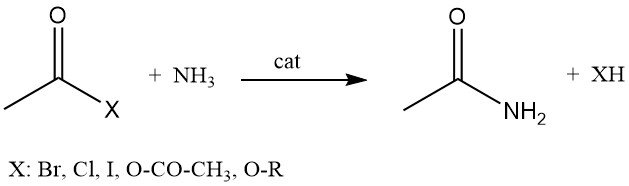
Acetamide can also be produced by the reaction of acetyl halides, acetic anhydride, or alkyl acetates with ammonia.
An alternative method for manufacturing acetamide is the hydration of acetonitrile in the presence of acid or base catalysts. This process is widely used because acetonitrile is produced as a by-product in the manufacture of acrylonitrile.
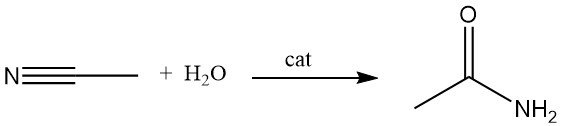
Copper-catalyzed hydration of acetonitrile can achieve conversion efficiencies up to 83%. Certain microorganisms are also capable of catalyzing this reaction near room temperature.
4. Uses of acetamide
Acetamide is used as a solvent and as a plasticizer. It has a broad range of applications across multiple industries.
Acetamide inhibits acid buildup in printing inks, lacquers, explosives, and perfumes. It is used as a mild moisturizer and as a softening agent for leather, textiles, paper, and certain plastics.
Acetamide is also employed as an intermediate in the synthesis of pharmaceuticals, pesticides, and plastic antioxidants. Derivatives of acetamide, including substituted acetamide-containing thioureas, have been investigated for antiviral activity against herpes viruses.
Acetamide is applied as a plasticizer and stabilizer in polymer production. It has been studied as a cryoprotective agent and as a component in lubricants and fireproofing formulations.
Certain acetamide derivatives are applied as feeding behavior modifiers in agricultural studies.
In laboratory research, acetamide is often used as a model compound for studying the structural and hydrogen-bonding properties of amides.
5. Toxicology of acetamide
Acetamide has been used as a source of nonprotein nitrogen in ruminants, such as sheep and dairy cattle. Dietary levels of approximately 2–3% appear non-toxic. Buffering the diet with dibasic acids allows higher intake by scavenging ammonia released during digestion.
The International Agency for Research on Cancer (IARC) classifies acetamide as a possible human carcinogen (Group 2B).
Chronic oral exposure in rats demonstrated hepatocarcinogenicity: 1-year administration of 2.36% acetamide in the diet resulted in liver cancer. Additional studies revealed hepatotoxic and hematopoietic effects.
The precise mechanism of acetamide toxicity remains unclear. Its profile differs from dimethylacetamide and appears related to hydroxylamine formation from its primary metabolite, acetohydroxamic acid.
Acetamide does not show developmental toxicity. Reproductive studies in rodents have not indicated altered spermatogenesis, hormonal changes, or damage to accessory reproductive organs.
In mice, oral administration of acetamide produced benign and malignant liver tumors. Male mice also showed increased incidence of malignant lymphomas. Overall, these findings support the classification of acetamide as a hepatocarcinogen in rodents with possible carcinogenic potential in humans.
References
1. Wagner, F.S., Jr. (2002). Acetamide. In Kirk-Othmer Encyclopedia of Chemical Technology, (Ed.). https://doi.org/10.1002/0471238961.0103052023010714.a02.pub2
2. Le Berre, C., Serp, P., Kalck, P. and Torrence, G.P. (2014). Acetic Acid. In Ullmann’s Encyclopedia of Industrial Chemistry, (Ed.). https://doi.org/10.1002/14356007.a01_045.pub3
3. Mohammadi, S.; Foroumadi, A. Acetamide. In *Encyclopedia of Toxicology*, 4th ed.; Wexler, P., Ed.; Academic Press, 2024; pp 39–42. DOI: 10.1016/B978-0-12-824315-2.01089-7
