1-Propanol: Properties, Reactions, Production and Uses
What is 1-propanol?
1-Propanol, also called n-propyl alcohol or propan-1-ol, is a primary alcohol with the chemical formula CH3CH2CH2OH. It is a clear, colorless liquid with a characteristic alcoholic odor and is miscible with water, ethanol, and ether.
1-Propanol is a structural isomer of isopropyl alcohol, but the latter has greater industrial significance due to its widespread use as a solvent and disinfectant.
In nature, 1-propanol is found in fusel oils and is formed as a minor product of fermentation and during the spoilage or decomposition of vegetable matter.
Table of Contents
1. Physical properties of 1-propanol
1-Propanol is a clear, colorless liquid with a characteristic alcoholic odor. It is completely miscible with water and readily soluble in many organic solvents, including ethers, esters, acids, ketones, and other alcohols.
The physical properties of n-propanol are summarized in Table 1.
| Property | Value |
|---|---|
| CAS Registry Number | 71-23-8 |
| Chemical formula | C3H8O |
| Molar mass, g/mol | 60.09 |
| Freezing point, °C | −126.2 |
| Boiling point, °C | 97.20 |
| Vapor pressure, kPa | at 20 °C: 1.987 at 40 °C: 6.986 at 60 °C: 20.292 at 80 °C: 50.756 |
| Antoine equation (2–120 °C, t in °C) | log P(kPa) = 6.97257 − 1499.21 / (204.64 − t) |
| Vapor density (air = 1) | 2.07 |
| Density at 20 °C, g/cm3 | 0.80375 |
| Francis equation (−21 to 180 °C, t in °C) | Density = 0.8813 + (5.448 × 10-4 t) − 21.536 / (313.09 − t) |
| Refractive index, nD20 | 1.38556 |
| Viscosity at 20 °C, mPa·s | 2.256 |
| Surface tension at 20 °C, mN/m | 23.75 |
| Critical temperature, °C | 263.65 |
| Critical pressure, kPa | 5169.60 |
| Critical density, g/cm3 | 0.275 |
| Heat capacity (liquid, 25 °C), J/(mol·K) | 141 |
| Heat of vaporization, kJ/mol | at 25 °C: 47.53 at 97.20 °C: 41.78 |
| Heat of combustion (liquid, 25 °C), kJ/mol | 2033 |
| Heat of formation (vapor, 25 °C), kJ/mol | −254.7 |
| Flash point (Tag open cup), °C | 28.9 |
| Autoignition temperature, °C | 371.1 |
| Explosive limits in air, vol % | Lower: 2.2 Upper: 14.0 |
| Electrical conductivity at 25 °C, S | 2 × 10-8 |
2. Chemical reactions of 1-propanol
The chemistry of 1-propanol is typical of low molecular weight primary alcohols. It undergoes a variety of reactions characteristic of the hydroxyl functional group, including oxidation, esterification, amination, dehydration, and etherification. In biological systems, 1-propanol is readily degraded and is considered one of the most easily biodegradable alcohols.
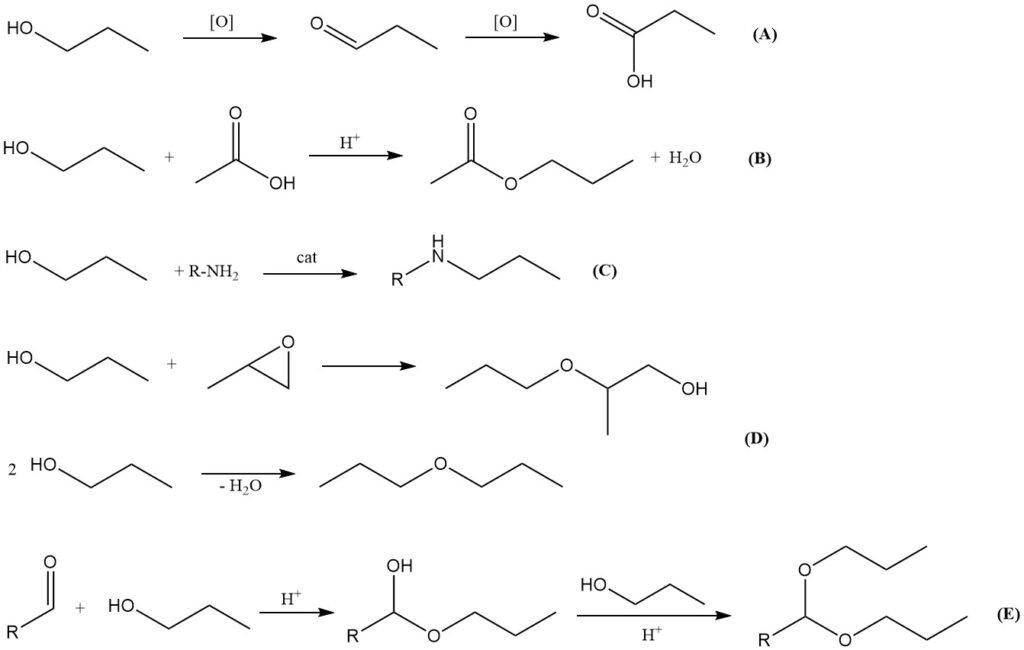
Oxidation (A)
Oxidation of 1-propanol proceeds first to the corresponding aldehyde, propanal, and with further oxidation to propionic acid. Partial oxidation can be achieved using air in the presence of metal-based catalysts such as copper chromite, chromium(VI) oxides, or pyridinium salts.
Esterification (B)
Like other alcohols, 1-propanol reacts with organic and inorganic acids to form esters. Reaction with acetic acid in the presence of strong acid catalysts (e.g., sulfuric acid, p-toluenesulfonic acid, methane sulfonic acid, or ion-exchange resins) yields n-propyl acetate, an important solvent used in coatings, inks, and the fragrance industry.
Transesterification with methyl or ethyl acetate can also be used to produce n-propyl acetate.
Amination (C)
1-Propanol can undergo reductive amination with ammonia or amines under elevated temperature and pressure in the presence of transition metal catalysts such as nickel, cobalt, or molybdenum. This reaction produces propylamine derivatives, although these are of less industrial importance compared to the corresponding isopropylamine derivatives.
Etherification and dehydration (D)
Reaction of 1-propanol with alkylene oxides (e.g., ethylene oxide, propylene oxide) yields glycol ethers, which are widely used as solvents. Dehydration of 1-propanol can produce di-n-propyl ether using solid acid catalysts. Dehydration to propene is also possible but has no practical industrial significance.
Acetal formation (E)
Like other primary alcohols, 1-propanol reacts with aldehydes to form hemiacetals, which can be further converted to acetals in the presence of acid catalysts under dehydrating conditions. Acetals derived from 1-propanol serve as intermediates in pharmaceutical synthesis.
3. Industrial production of 1-propanol
1-Propanol is mainly produced industrially by the hydroformylation (Oxo process) of ethylene to form propanal (propionaldehyde), followed by catalytic hydrogenation to the alcohol.
A vapor-phase oxidation process of propane was once used, operated by Celanese at Bishop, Texas, but this route was discontinued in 1973. Since then, OXO technology has been the predominant method in the United States and Europe.
In South Africa, Sasol produces 1-propanol through Fischer–Tropsch synthesis. Attempts to manufacture 1-propanol by anti-Markovnikov hydration of propylene have been investigated but have not reached commercial application.
3.1. Production of 1-propanol by hydroformylation and hydrogenation
Commercial production of 1-propanol by hydroformylation (oxo process) is a two-step sequence. Ethylene is first converted to propanal by hydroformylation with synthesis gas (1), followed by catalytic hydrogenation of propanal to 1-propanol (2).
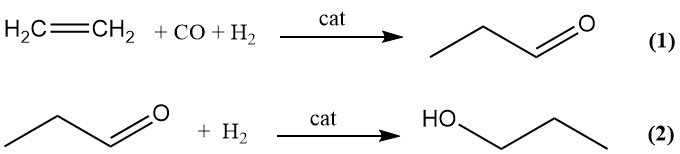
Minor byproducts of the hydroformylation step include propane, 1-propanol, and heavy ends formed by aldol condensation. Transition-metal carbonyl complexes of cobalt, iron, nickel, rhodium, and iridium can catalyze the oxo reaction, but only cobalt and rhodium are used commercially.
In the United States, 1-propanol is manufactured by oxo technology at Texas Eastman, Union Carbide, and Hoechst Celanese. Texas Eastman originally operated with cobalt-based HCo(CO)4 catalysts, later adopting a phosphine-modified rhodium system in 1989. In Europe, production is carried out by Hoechst AG and BASF AG.
The rhodium–triphenylphosphine catalyst has largely replaced cobalt catalysts due to higher reaction rates, improved stability, reduced operating pressures, and fewer by-products. Rhodium carbonyls are suitable for plants originally designed for cobalt catalysts.
Although rhodium requires higher pressures, its activity is greater and it produces fewer high-boiling fractions. At Hoechst AG in Germany (Werk Ruhrchemie), rhodium carbonyls are used for propanal synthesis, which is then hydrogenated to 1-propanol. Rhodium carbonyls are reported to be 100–1000 times more reactive than cobalt analogs.
Ethylene hydroformylation with rhodium catalysts is conducted at 90–120 °C, 2.17–3.55 MPa, H2:CO ratios of 1:1–3:1, rhodium concentrations of 1–10 mM, and triphenylphosphine concentrations of 0.1–0.4 M. Under these conditions, propanal yield reaches 98–99%, with 0.5–1.0% conversion to ethane and heavy ends.
Catalyst activity is suppressed by halogen-containing rhodium sources, as halogenated complexes display poor hydroformylation performance. The most active catalyst precursor is hydridocarbonyltris(triphenylphosphine)rhodium, HRhCO\[P(C6H5)3]3, which generates active species by dissociation of a triphenylphosphine ligand.
Excess triphenylphosphine suppresses catalyst deactivation caused by phenyl migration from phosphorus to rhodium, which otherwise leads to inactive rhodium–phosphide clusters.
The hydrogenation of propanal to 1-propanol is an established process. Nickel-based catalysts, such as Raney nickel or supported nickel, and copper–chromium oxide catalysts are commonly used. Both vapor-phase and liquid-phase hydrogenation methods are employed.
Liquid-phase operation is conducted at 2.17–4.24 MPa and 100–170 °C. Vapor-phase hydrogenation typically operates below 790 kPa. Fixed-bed, slurry-bed, or trickle-bed reactor configurations are used. Yields to 1-propanol above 95% are achieved.
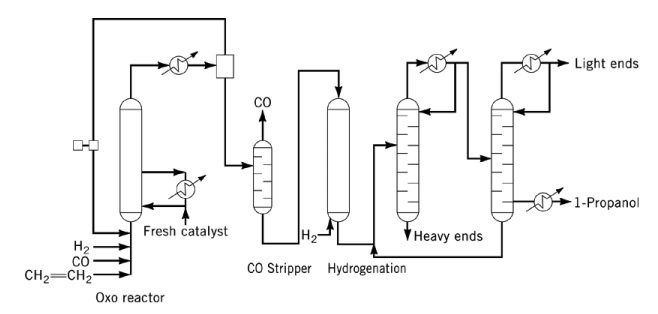
By-products include acetals, ethers, esters, and diols. Both CO and triphenylphosphine act as catalyst poisons and must be removed from the feed prior to hydrogenation.
In industrial operation, vapor of propanol is stripped from the oxo reactor effluent using excess synthesis gas, followed by condensation and CO removal prior to hydrogenation. The resulting crude 1-propanol is purified in a two-column distillation system.
When cobalt carbonyl or rhodium carbonyl catalysts are used, liquid withdrawal of propanal is necessary due to higher reactor pressures, and additional provisions for catalyst recovery and handling are required.
3.2. Production of 1-propanol by the Sasol Fischer-Tropsch process
Sasol produces 1-propanol as a secondary product of the Fischer–Tropsch synthesis. In this process, coal is gasified in Lurgi fixed-bed reactors to generate synthesis gas (CO and H2). The gas stream is separated from condensable components, purified, and introduced into the Sasol Synthol unit.
Within fluidized-bed reactors, the gas is contacted with a finely divided iron-based catalyst, where the highly exothermic Fischer–Tropsch reaction yields a mixture of hydrocarbons and oxygenated compounds.
The condensates from the reactors separate into a hydrocarbon fraction and an aqueous phase. The aqueous stream contains a mixture of alcohols and ketones. Most of the alcohols are blended into high-octane gasoline, while selected alcohol fractions undergo distillation for recovery of pure products.
From these operations, 1-propanol and ethanol are isolated in a multiunit separation system with an overall annual capacity of approximately 25,000–30,000 t.
4. Uses of 1-propanol
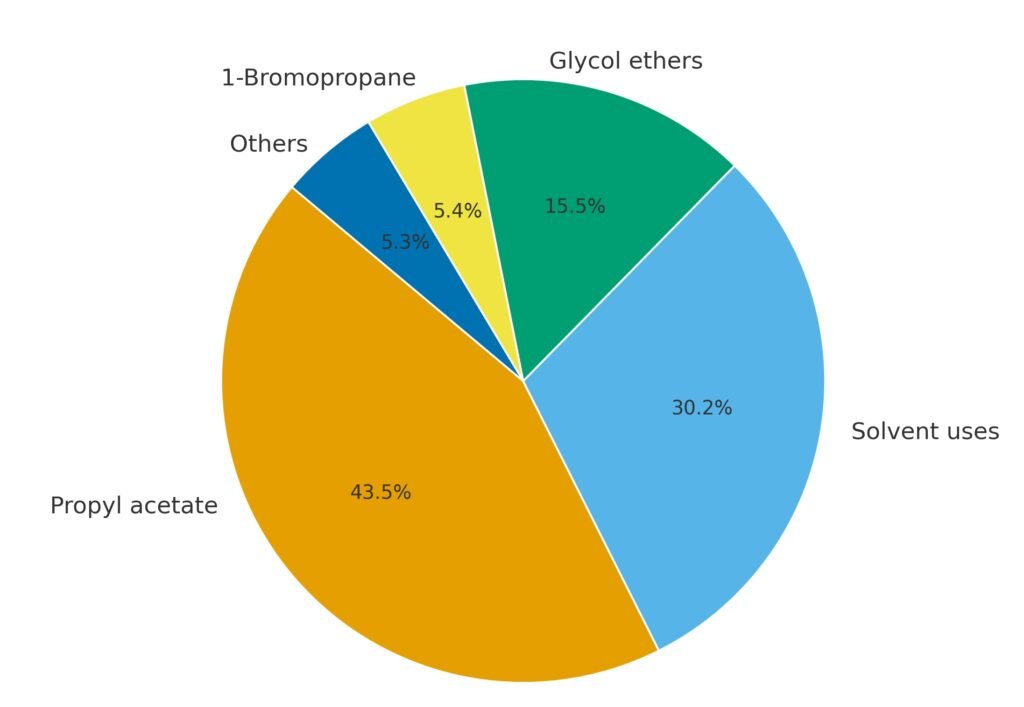
1-Propanol is used mainly as a solvent and as a chemical intermediate. The most important derivative is n-propyl acetate, which accounts for the largest share of global consumption.
1-Propanol is widely used as a solvent in flexographic and rotogravure printing inks, particularly for printing on polyolefin and polyamide films. Compared with ethanol and 2-propanol, it provides advantages in print quality and drying behavior. It is also used as a solvent in paints, coatings, cosmetics, pesticides, and insecticides.
In Europe, 1-propanol has become increasingly important as an ingredient in hand and surface disinfectants because of its high biocidal activity against bacteria, fungi, and viruses.
A major portion of 1-propanol is converted to n-propyl acetate, used as a solvent in inks, lacquers, cellulose derivatives, waxes, and insecticide formulations. Other esters, such as n-propyl propionate, are used in coatings and are considered replacements for n-butyl acetate due to improved odor characteristics.
1-Propanol is a feedstock for the production of glycol ethers, such as ethylene glycol monopropyl ether, diethylene glycol monopropyl ether, propylene glycol monopropyl ether, and dipropylene glycol monopropyl ether. These solvents combine high solvency with water compatibility and are used in coatings and cleaners.
In Europe, a significant fraction of 1-propanol is converted to propylamines, which are intermediates in the manufacture of herbicides and pharmaceuticals.
1-Propanol is a precursor to 1-bromopropane, a solvent used as a substitute for methylene chloride and ozone-depleting compounds.
Minor applications include use as a flavor and fragrance ingredient, a cosolvent in pesticide formulations, and as a feed additive.
5. Toxicology of 1-propanol
1-Propanol is classified as a flammable liquid with a flash point below 38 °C. It is regulated as a hazardous substance by the Occupational Safety and Health Administration (OSHA) under 29 CFR 1910.1200.
Toxicity studies show that 1-propanol is only slightly toxic to animals (Table 2). It produces negative results in the Ames test and in the Mouse Lymphoma Forward Mutation Assay, indicating no mutagenic potential.
| Administration method / Parameter | Value |
|---|---|
| Oral dose, rats, LD50 | 1.9 g/kg |
| Dermal, rabbits, LD50 | 5.4 g/kg |
| Inhalation, rats, LC50 | 24,000 ppm |
| ACGIH TLV, TWA | 200 ppm |
| Hazard ratings (NFPA: Health, Flammability, Reactivity) | 1, 3, 0 |
| Hazard ratings (HMIS: Health, Flammability, Reactivity) | 2, 3, 0 |
| Notes: LD50 = median lethal dose. LC50 = median lethal concentration. TLV = Threshold Limit Value, TWA = time-weighted average. | |
The National Toxicology Program (NTP) and the International Agency for Research on Cancer (IARC) do not classify 1-propanol as a carcinogen. Acute exposure can cause eye irritation or burns, while repeated skin contact may lead to dermatitis. Inhalation of excessive vapor concentrations can irritate the eyes and respiratory tract, and very high concentrations may cause narcotic effects.
In the United States, 1-propanol is listed under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) with a reportable spill quantity of 100 lb (45.4 kg) per day.
It is also included in atmospheric emission standards under 40 CFR 60.489, which require control of volatile organic compound emissions from manufacturing equipment. Several U.S. states list 1-propanol under right-to-know regulations.
In food and pesticide regulations, 1-propanol is permitted as a flavoring substance and adjuvant under 21 CFR 172.515 and is exempted from tolerance requirements when used as a solvent or co-solvent in pesticide formulations.
References
1. Unruh, J.D. and Pearson, D. (2000). n-Propyl Alcohol. In Kirk-Othmer Encyclopedia of Chemical Technology, (Ed.). https://doi.org/10.1002/0471238961.1618151621141821.a01
2. Klabunde, J., Bischoff, C. and Papa, A.J. (2025). Propanols. In Ullmann’s Encyclopedia of Industrial Chemistry. https://doi.org/10.1002/14356007.a22_173.pub3
