Production Processes of Titanium Dioxide: a Complete Overview
Commercial titanium dioxide is manufactured via two distinct industrial methods: the sulfate process and the chloride process.
The sulfate process, an older technique, starts with the digestion of titanium-containing raw materials, such as ilmenite or titanium slag (→ detailed article about raw materials), in concentrated sulfuric acid. This reaction occurs at 150–220 °C, yielding a black liquor that contains dissolved titanium, iron, and various impurities.
Subsequent hydrolysis of the titanyl sulfate solution precipitates relatively pure titanium oxide hydrate (TiO(OH)2 or TiO2 dihydrate) and further purification steps eliminate the remaining impurities. The titanium oxyhydrate then undergoes calcination, grinding, and usually, inorganic compound coating.
The chloride process involves chlorinating titanium-containing raw materials, including ilmenite, leucoxene, rutile, titanium slag, or anatase, at temperatures ranging from 700–1200 °C. The resulting titanium tetrachloride (TiCl4) is then oxidized at 900–1400 °C to form titanium dioxide, which is subsequently ground and coated with inorganic compounds.
These two processes are exclusively employed for producing titanium dioxide pigments. Other manufacturing methods exist for TiO2 without pigment properties, primarily for titanium dioxide nanoparticle synthesis. Examples include the hydrolysis of titanium alcoholates or the pyrolytic reaction of TiCl4 with water (the aerosil process).
Table of Contents
1. Production of Titanium Dioxide by the Sulfate Process
The production of titanium dioxide by the sulfate process involves several sequential stages, as illustrated in Figure 1.
1.1. Grinding
Titanium-containing raw materials undergo drying to less than 0.1% moisture content to prevent premature reactions and heating when mixed with sulfuric acid. Materials are then ground in ball or bowl mills to obtain a mean particle size below 40 μm.
1.2. Digestion
Batch digestion is the standard procedure where ground raw materials (ilmenite, titanium slag, or mixtures) are mixed with 80–98% H2SO4.
The reaction can be initiated by adding oleum to 80% sulfuric acid or by adding water and/or steam to raw material suspension in concentrated sulfuric acid. In both cases, mixing enthalpy triggers an exothermic chemical digestion which reaches a maximum temperature of approximately 200 °C or higher.
The H2SO4 to raw material ratio is adjusted to obtain a ratio of 1.8 to 2.2 in weight of sulfuric acid to titanium dioxide in the resulting black liquor (acid number).
The reaction in the digestion vessel (f) begins with the addition of water, dilute sulfuric acid, oleum, or steam. An initial temperature increase to 50–70 °C is caused by the heat of acid hydration. Subsequent exothermic sulfate formation drives the temperature to 170–220 °C.
The added and reaction-generated water completely evaporates to form a solid digestion cake. External heating may be required for dilute acid or sparingly soluble raw materials.
After reaching maximum temperature, the solid reaction mixture requires 1–12 hours of maturation, with the duration depending on the raw material. Maturation maximizes the solubility of titanium-containing components. Air agitation during the temperature increase positively influences digestion yield.
Continuous digestion processes exist. One established method involves continuously feeding an ilmenite and water mixture with sulfuric acid into a double-paddle screw conveyor. A crumbly cake forms after a short residence time (less than 1 hour). This process is suitable for highly reactive raw materials.
1.3. Redigestion
Due to high costs of titaniferous raw materials, attempts have been made to reuse solid digestion residue (containing 40–65% titanium dioxide). Historically, these developments did not achieve large-scale implementation because of the complex and expensive equipment or processing conditions.
A redigestion process using standard equipment and technology was proposed in 2001.
1.4. Dissolution and reduction
The digestion cake is dissolved in cold water or recycled dilute acid at a temperature below 85 °C to prevent premature hydrolysis, particularly with ilmenite products. Air is introduced for agitation during dissolution. With ilmenite, the concentration of titanium dioxide solution is 8–12 wt%, and with slag, it ranges from 13 to 18 wt%.
The trivalent iron (Fe3+) is hydrolyzed together with titanium compounds and adheres to the titanium oxide hydrate. Therefore, all Fe3+ must be reduced to Fe2+ using scrap iron during ilmenite product dissolution or in a subsequent step.
To prevent reoxidation of iron during subsequent processing, a small amount of Ti3+ is maintained until hydrolysis completion. Ti3+ is formed by using excess scrap iron during the reduction of Fe3+. Ti4+ can also be partially reduced to Ti3+ under optimized conditions. This concentrated Ti3+ solution is then added to the main reaction solution.
Solutions obtained from titanium slag already contain higher amounts of Ti3+, which must be decreased by oxidation with atmospheric oxygen to prevent yield loss during hydrolysis. Ti3+ present in slag digestion partially reduces sulfuric acid to SO2 or H2S, which require extensive off-gas cleaning, especially if the slag contains heavy metals.
Mixed digestion of ilmenite and titanium slag is possible, in which the Ti3+ from the slag reduces all Fe3+ to Fe2+. This can also be achieved by mixing dissolved products from separate ilmenite and titanium slag digestions.
1.5. Clarification
All undissolved solid material must be thoroughly removed from the solution to avoid hydrolysate contamination. The most economical method involves preliminary settling in a thickener (g) followed by filtration of the sediment using a rotary vacuum drum filter (h) or a filter press.
The thickener supernatant is also passed through filter presses (i) to remove residual fines. Due to poor filtering properties, the rotary filter typically operates as a precoat filter. The addition of chemical can promote flocculation and sedimentation of fines in the thickener.
Single-stage clarification using automated filter presses has also been proposed.
1.6. Crystallization
After Fe3+ reduction , solutions from slag digestion contain 5–6 wt% FeSO4 and those from ilmenite digestion contain 16–20 wt% FeSO4. Ilmenite solutions are cooled under vacuum to crystallize and separate FeSO4·7 H2O (copperas) (j) to reduce FeSO4 discharged with waste acid after hydrolysis. This increases the concentration of titanium dioxide in the solution by approximately 25%.
Copperas salt is separated by filtration or centrifugation (k).
Iron sulfate is primarily used in water purification, as a chromate reducing agent for cement, and as a raw material for iron oxide pigments. It can also be dehydrated to form FeSO4·H2O or thermally decomposed into iron(III) oxide and sulfur dioxide.
1.7. Hydrolysis
Titanium oxide hydrate is precipitated by hydrolysis at 94–110 °C. Other sulfuric acid-soluble raw material components, mainly niobium as oxide hydrate, precipitate simultaneously. Hydrolysis is conducted in brick-lined, stirred tanks (n) with steam injection. The resulting hydrolysate lacks pigment properties.
Hydrolysate properties are significantly influenced by the flocculation degree of primary particles. Hydrolysate primary particle size is approximately 5 nm, while TiO2 pigment particle size is 200–300 nm. Several factors affect hydrolysate properties:
1. Hydrolysis of concentrated titanium sulfate solutions (170–230 g TiO2/L) is sluggish and incomplete without suitable nuclei, which accelerate the hydrolysis. Nuclei are mainly produced by the Mecklenburg or Blumenfeld method.
In the Mecklenburg method, colloidal titanium oxide hydrate is precipitated with sodium hydroxide at 100 °C. 1% of this hydrate is used as seed material.
In the Blumenfeld method, a small portion of the sulfate solution is hydrolyzed in boiling water, then added to the bulk solution. The number of nuclei influences hydrolysate particle size and flocculation characteristics.
2. Hydrolysate particle size and flocculation degree also depend on agitation intensity during Blumenfeld nuclei formation and the initial hydrolysis stage.
3. Titanium sulfate concentration greatly affects hydrolysate flocculation and is adjusted by vacuum evaporation, if necessary, to 170–230 g/L of titanium dioxide during hydrolysis. Lower concentrations result in coarser flocculates.
4. The acid number should be between 1.8 and 2.2. It significantly impacts TiO2 yield, hydrolysate particle size, and flocculate size. High acid numbers lead to lower yield and coarser hydrolysate. A normal hydrolysis period (3–6 hours) yields 93–96% titanium dioxide.
Other salt concentrations, especially FeSO4, affect hydrolysate properties. High concentrations typically result in finer flocculates.
5. The temperature mainly influences volume–time yield and hydrolysate purity.
Hydrolysate structure is complex. Nanocrystalline anatase primary particles exhibit a size of approximately 5–6 nm (electron micrographs, BET surface data). Conventional particle size determinations (e.g., laser scattering) show values from hundreds to thousands of nanometers, depending on dispersion intensity.
The primary particles aggregate into secondary particles of approximately 30 nm (Figure 3). These secondary particles form tertiary structures about 1 μm in size. Tertiary structures then form quaternary structures of a few micrometers.
Grinding or high-intensity ultrasonic dispersion can break down quaternary structures. Sufficient dispersion energy can partially break down tertiary structures.
During calcination, morphological transformations occur:
- At 300–600 °C, primary particles sinter. Former secondary particles form new primary particles of crystalline anatase, approximately 20 nm in size. This is a typical size for catalytic titanium dioxide.
- At 800–1000 °C, the 20 nm particles sinter. Original tertiary particles form new primary particles of crystalline rutile or anatase, approximately 200 nm in size. This is a typical size for pigment titanium dioxide. Original quaternary structures form pigment particle agglomerates, which are separated by a milling step.
1.8. Purification of the hydrolysate
After hydrolysis, the liquid phase contains 20–28% sulfuric acid and varying amounts of dissolved sulfates depending on the raw material. Titanium oxide hydrate is separated from the solution by filtration and is then washed with water or dilute acid. Even with acid washing, too many heavy metal ions remain adsorbed on the titanium oxide hydrate for white pigment production.
Most impurities can be removed by reduction (bleaching). The filter cake is slurried with 3–10% dilute acid at 50–90 °C and is then mixed with zinc or aluminum powder (q). Non-metallic reducing agents (e.g., HOCH2–SO2Na) can also be used for bleaching.
After a second filtration and washing, the titanium oxide hydrate has low concentrations of colored impurities. However, it still contains 5–10% chemisorbed sulfuric acid, which cannot be completely removed by washing. It is driven off by high-temperature heating.
1.9. Doping of the hydrate
To produce high purity titanium dioxide, the titanium oxide hydrate is calcined without additions to yield a relatively coarse TiO2 grade with rutile content dependent on heating temperature. To produce specific pigment grades, the titanium oxide hydrate must be treated with alkali-metal compounds and phosphoric acid before calcination (r).
Anatase pigments contain more phosphoric acid than rutile pigments. For rutile pigments, rutile nuclei (less than 10%, typically 1–5%) are added. ZnO, Al2O3, and/or Sb2O3 (less than 3%) are added to stabilize the crystal structure.
Nuclei (or rutile seed) are produced by converting purified titanium oxide hydrate to sodium titanate, which is washed free of sulfate and then treated with hydrochloric acid. Rutile nuclei can also be prepared by precipitation from titanium tetrachloride solutions with sodium hydroxide solution.
1.10. Calcination
Doped titanium oxide hydrate is filtered using rotary vacuum filters or filter presses (s) to remove water. Rotary vacuum filters achieve approximately 30–40% TiO2 solids content. Pressure rotary filters or automatic filter presses result in approximately 50% or higher TiO2 content.
Calcination occurs in rotary kilns (t), which are directly heated with gas or oil in counter-current flow. Approximately two-thirds of the total residence time (4–20 hours) is for material drying. Above approximately 500 °C, sulfur trioxide is driven off and partially decomposed to sulfur dioxide and oxygen at higher temperatures.
The product reaches a maximum temperature of 800–1100 °C depending on the pigment type, throughput, and kiln temperature profile. Rutile content, particle size, size distribution, and aggregate formation are highly sensitive to kiln operating regimes.
After exiting the kiln, the clinker can be indirectly cooled or directly air-cooled in drum coolers (u). Exhaust gas exit temperature from the kiln must exceed 300 °C to prevent sulfuric acid condensation in the piping.
Energy can be conserved by recirculating some gas to the kiln combustion chamber. This gas can be mixed with fuel gases as a partial air replacement or it can be used to concentrate the diluted acid. The gas is then transmitted to the waste-gas purification system.
1.11. Grinding
Agglomerates and aggregates in the clinker are reduced to pigment fineness by wet or dry grinding. Coarse size reduction should occur in hammer mills (Raymond mills) or roller mills prior to wet grinding in ball mills or sand mills (with addition of a dispersion agent).
The coarse fraction can be removed from the suspension by centrifugation or hydrocyclones and recycled to the mills. Hammer mills, cross-beater mills, roller mills, and particularly pendular and steam-jet mills are suitable for dry grinding.
Special grinding additives can be used as wetting agents during subsequent pigment treatment or to improve untreated pigment dispersibility.
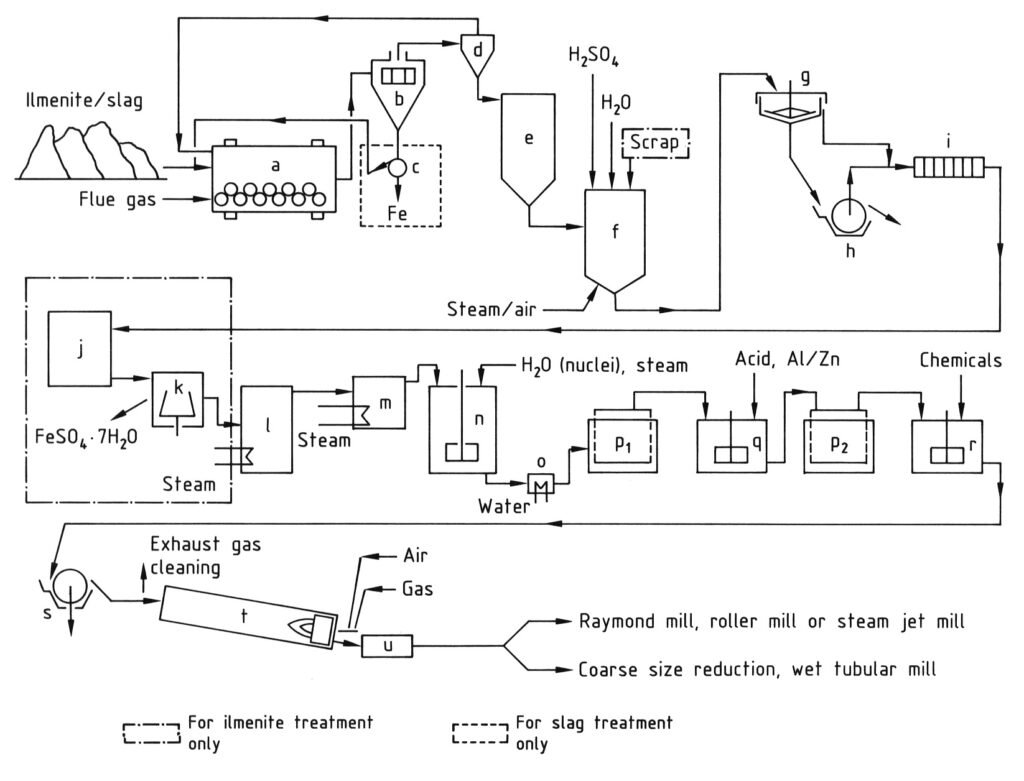
a) Ball mill/dryer; b) Screen; c) Magnetic separator; d) Cyclone; e) Silo; f) Digestion vessel; g) Thickener; h) Rotary filter; i) Filter press; j) Crystallizer; k) Centrifuge; l) Vacuum evaporator; m) Preheater; n) Stirred tank for hydrolysis; o) Cooler; p) Moore filters; q) Stirred tank for bleaching; r) Stirred tank for doping; s) Rotary filter for dewatering; t) Rotary kiln; u) Cooler
2. Production of Titanium Dioxide by the Chloride Process
The production of titanium dioxide pigment by the chloride process is illustrated in Figure 2.
2.1. Chlorination
Titanium in raw materials is converted to titanium tetrachloride in a reducing atmosphere. Calcined petroleum coke with extremely low ash content is used as the reducing agent. Due to its low volatiles content, HCl formation is minimal. Titanium dioxide reacts exothermically according to the following equation:
TiO2 + 2 Cl2 + C → TiCl4 + CO2
As temperature increases, an endothermic reaction occurs between carbon dioxide and carbon to form carbon monoxide. Oxygen must be co-fed with chlorine to maintain the reaction temperature between 800 and 1200 °C. Coke consumption is 250–300 kg per ton of titanium dioxide. If CO2-containing chlorine from TiCl4 combustion is used, the coke consumption increases to 350–450 kg.
Older fixed-bed chlorination is obsolete. In tthis process ground titanium raw material is mixed with petroleum coke and a binder. Briquettes were formed. Chlorination was performed at 700–900 °C in brick-lined reactors.
Fluidized-bed chlorination began in 1950. Titanium raw material (sand-like particle size) and petroleum coke (mean particle size approximately five times that of titanium dioxide) react with chlorine and oxygen. This occurs in a brick-lined fluidized-bed reactor at 800–1200 °C.
Raw materials must be very dry to prevent HCl formation. Chlorine conversion is 98–100%. Titanium conversion in the raw material is 95–100%. The efficiency of this process depends on reactor design and gas velocity. Losses are primarily due to dust entrainment.
Magnesium chloride and calcium chloride can accumulate in the fluidized-bed reactor due to low volatility. Silicon dioxide and zirconium silicate also accumulate because they chlorinate very slowly at process temperatures. All other raw material constituents volatilize as chlorides in the reaction gases.
The ceramic lining of the fluidized-bed reactor is susceptible to abrasion and corrosion. If chlorination is interrupted, there is a risk that the raw materials may sinter, which can prevent subsequent fluidization.
2.2. Gas Cooling
Reaction gases are quenched with liquid TiCl4 and the cooling can be indirect or direct (d).
The chlorides of the other components crystallize and tend to build up on cooling surfaces, particularly large quantities of iron(II) and iron(III) chlorides formed by the chlorination of ilmenite. In this initial stage, reaction gases are cooled to below 300 °C to separate the accompanying chlorides from TiCl4 by condensation or sublimation (e).
The gas mainly contains TiCl4. It is cooled below 0 °C to condense most of the TiCl4 (f). The small amounts of TiCl4 and chlorine remaining in the exhaust gas (CO2, CO, and N2) are removed by alkali scrubbing (t).
2.3. Purification of TiCl4
The solid chlorides and entrained dust are separated from TiCl4 by simple evaporation (distillation) (j). Dissolved chlorine is removed by heating or reduction with metal powders (Fe, Cu, or Sn).
Removing vanadium tetrachloride and vanadium oxychloride from TiCl4 by distillation is more complex because their boiling points are very close. Therefore, they are reduced to form solid, low-valence vanadium chlorides (i).
Numerous reducing agents have been recommended. Examples include copper, titanium trichloride, hydrogen sulfide, hydrocarbons, soaps, fatty acids, and amines. After subsequent evaporation (j), titanium chloride should contain less than 5 ppm vanadium.
Phosgene and SiCl4 can be removed by fractional distillation.
2.4. Oxidation of TiCl4 and recovery of TiO2
The titanium dioxide pigment and chlorine are formed by the reaction of titanium tetrachloride with oxygen at 900–1400 °C. Purified TiCl4 is vaporized (k) and the vapor is indirectly heated to approximately 500–1000 °C (l).
TiCl4 + O2 → 2 Cl2 + TiO2
This reaction is moderately exothermic and requires a high reaction temperature. Therefore, oxygen must be heated to over 1000 °C (m) using an electric plasma flame, by reacting part of the oxygen with carbon monoxide or another fuel.
Hot TiCl4 and hot oxygen (110–150% stoichiometric amount) are fed separately into a reaction chamber. They must be mixed rapidly and completely to ensure a high reaction rate. Many different reactor designs have been proposed and used.
Similar considerations apply to the cooling unit (o). The pigment must be rapidly cooled to below 600 °C using cooling zones of various geometries. Material sticking to walls is usually prevented by adding abrasive particles such as sand, coarse TiO2 particles, sodium chloride, or other materials.
In the production of titanium dioxide by the chloride process, the design of the oxidation reactor and appropriate materials, along with processing parameters, are the most important. Numerous patents demonstrate extensive optimization efforts for this process step. Only a few companies possess the industrial-scale knowledge for this process.
The reaction mixture, comprising gases (Cl2, O2, CO2) and titanium dioxide pigment, can be further cooled during solid particle separation from the pigment, either indirectly or directly (p). TiO2 is separated from the gas stream by gravity separation, cyclones, filters, or combinations of devices.
The gas stream is recycled to the oxidation reactor’s cooling zone (o) and to the chlorination process. It is recycled as an oxygen-containing chlorine via the liquefaction unit (u) or directly as a gas. Chlorine adsorbed on the titanium dioxide pigment can be removed by heating, flushing with nitrogen or air, or wet chemical treatment.
Wet separation by quenching the pigment-containing gas mixture (Cl2, O2, and CO2) in water has not found large-scale application.
Titanium dioxide pigment quality (particle size and size distribution) depends on reaction temperature, additives, excess oxygen, and rheological conditions in the reactor. Sophisticated conditions must be established for each reactor.
The presence of water and/or alkali compounds during TiCl4 combustion generates nuclei, which promote controlled formation of finely divided pigment particles with high scattering power. Additives can be directly added to oxygen or can be produced by combustion of hydrogen-containing materials.
The presence of aluminum chloride promotes rutile formation and finer pigment division. It is added in amounts of 0.2–2.0 wt%. The addition of PCl3 and SiCl4 suppresses rutile formation, which yields anatase pigment. However, this type of pigment has not found major relevance for the chloride process.
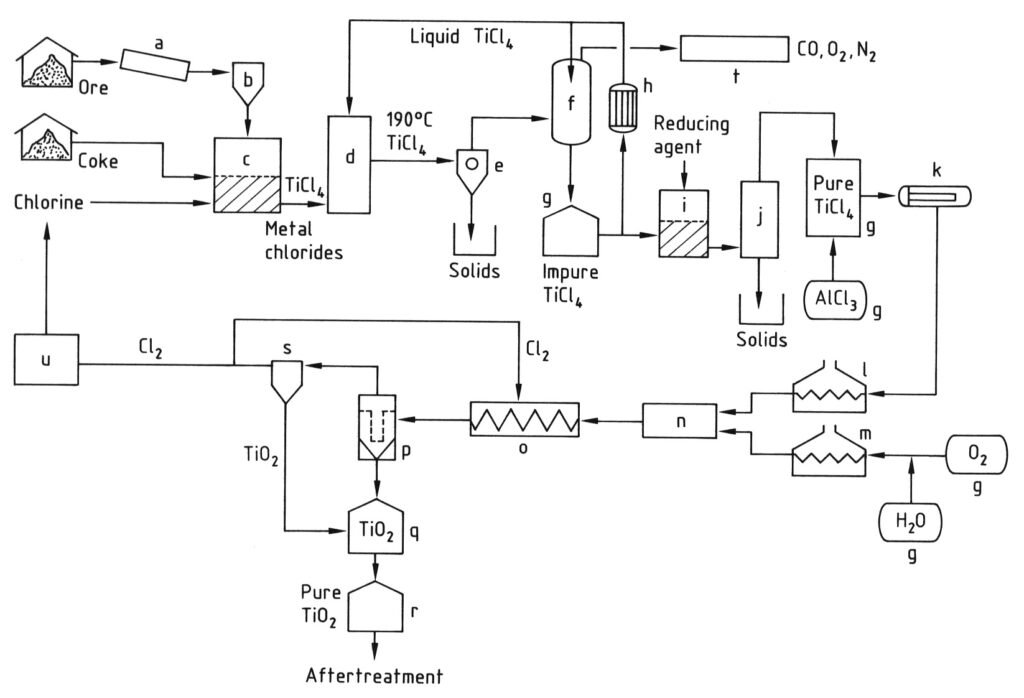
a) Mill; b) Silo; c) Fluidized-bed reactor; d) Cooling tower; e) Separation of metal chlorides; f) TiCl4 condensation; g) Tank; h) Cooler; i) Vanadium reduction; j) Distillation; k) Evaporator; l) TiCl4 superheater; m) O2 superheater; n) Burner; o) Cooling coil; p) Filter; q) TiO2 purification; r) Silo; s) Gas purification; t) Waste-gas cleaning; u) Cl2 liquefaction
3. Chloride Process vs. Sulfate Process for Titanium Dioxide Production
The global production capacity for titanium dioxide is nearly split between the two main processes. Currently, the sulfate process accounts for approximately 47% of capacity, while the chloride process holds about 53%.
Actual production and sales figures are more in favor of the chloride process, as some sulfate plants, particularly in China, operate below their full capacity. The clear upward trend for the chloride process, which peaked around 57% in 1996, has recently plateaued due to the significant increase in new sulfate process capacity in China.
TiO2 pigments produced by the chloride process generally have superior brightness and a more neutral masstone compared to the sulfate process. They often have better scattering power and enhanced durability. However, for many applications, these two types of pigments are interchangeable.
In certain uses, such as for fibers and printing inks, sulfate pigments may offer superior properties. This is often attributed to their less abrasive nature. Titanium dioxide pigments for demanding applications almost always undergo inorganic aftertreatment.
Anatase pigments and technical titanium dioxides, which require the anatase modification, including those for catalysts, cosmetic nanoparticles, photocatalytic uses, and electroceramics, are produced exclusively by the sulfate process.
The chloride process is often considered to have less environmental impact. This assessment focuses on the volume of onsite waste generated by the titanium dioxide plant, excluding waste from raw material upgrading.
Some major sulfate plants have achieved complete weak acid recycling, and most or all iron-containing byproducts are sold or processed. In contrast, waste from many chloride plants is still disposed of in landfills or deep wells.
| Pigment Class | TiO2 (min.), wt% | Water-soluble salts, wt% | Volatiles (max.), wt% |
|---|---|---|---|
| Anatase A 1 (Type A) | 98 | 0.6 | 0.5 |
| Anatase A 2 | 92 | 0.5 | 0.8 |
| Rutile R 1 (Type R) | 97 | 0.6 | 0.5 |
| Rutile R 2 | 90 | 0.5 | 1.5 |
| Rutile R 3 | 80 | * | * |
4. Waste Management in Titanium Dioxide Production
4.1. Aqueous Waste
The sulfate process consumes 2.4–3.5 tons of concentrated sulfuric acid per ton of titanium dioxide produced, depending on the raw material. During processing, some sulfuric acid converts to sulfates, primarily iron(II) sulfate. The remaining sulfuric acid is obtained as free sulfuric acid (weak acid).
Fractional filtration and washing of the hydrolysate suspension yield 70–95 wt% SO42- in a weak acid fraction, which contains approximately 20–25% free acid. The remaining 5–30% sulfate is highly diluted with wash water.
Historically, direct discharge of acid into open or coastal waters was common practice. The weak-acid problem generated significant political controversy. Consequently, the European Community mandated cessation of weak acid discharge into open waters by 1993.
European titanium dioxide producers developed various effluent-treatment processes to meet environmental regulations. Key processes include gypsum precipitation from weak acid and concentration/recovery of free and bound acid.
In the gypsum process, acid effluent is initially treated with finely divided CaCO3 to precipitate white gypsum. White gypsum is filtered, washed, and dried. It is then used for plasterboard manufacturing. In a second stage, residual metal sulfates in the filtrate are precipitated as metal hydroxides along with additional gypsum by adding calcium hydroxide. This mixture called red gypsum has to be landfilled.
Production of iron oxide pigments from iron sulfate solution, obtained after partial neutralization of weak acid with CaCO3 or metallic iron, has also been suggested.
In the recycling process, both free and bound sulfuric acid (as metal sulfates) can be recovered from weak acid by concentration and thermal cracking in a fluidized-bed furnace (k, Figure 3). The process has two stages:
- Concentration and recovery of free acid via evaporation.
- Separation and thermal decomposition of metal sulfates, and sulfuric acid production from the resulting sulfur dioxide.
Due to energy requirements, only acid containing over 20% sulfuric acid is economically recoverable by evaporation. Weak acid is concentrated from approximately 20–25% to about 28% with minimal heat consumption by using waste heat from contact process sulfuric acid production or from titanium dioxide calcination kiln waste gases.
After preliminary evaporation, further concentration occurs in vacuum evaporators. Water vapor pressure strongly decreases with increasing H2SO4 concentration. Evaporation yields a suspension of metal sulfates in 60–70% sulfuric acid (stage 1 in Figure 3).
The suspension is cooled to 40–60 °C in a series of stirred tanks (stage 2, d) to produce a product with good filtering properties and an acid suitable for recycling to the digestion process. Filtration (stage 3, e) is usally carried out with pressure filters to provide a filter cake with low residual liquid content.
The concentration requirements of the acid recycled to the digestion process depend on the titanium-containing raw material quality. For raw materials with high titanium content, the 65–70% sulfuric acid separated from metal sulfates must be further concentrated to 80–87% acid (stage 5).
Concentration can be performed in steam-heated vacuum evaporators. Alternatively, heat from titanium dioxide calcination kilns can be used. Cooling the acid after this concentration process yields a metal sulfate suspension in sulfuric acid. This acid can be directly used for raw material digestion.
Metal sulfates recovered and separated from sulfuric acid in stage 3 (usually by filtration, thus called “filter salt”) contain approximately 15–30% sulfuric acid with a concentration of 60 to 70%. They can be converted to a disposable material by reaction with calcium compounds.
Thermal decomposition of metal sulfates to form metal oxides, sulfur dioxide, water, and oxygen is energy-intensive. However, it is ecologically advantageous. The energy requirement is approximately 4 × 109 J per ton of filter cake. Thermal decomposition occurs at 850–1100 °C in a fluidized-bed furnace (stage 6).
Energy is supplied by coal, pyrites, or sulfur. Sulfur dioxide formed during the thermal decomposition is purified by standard methods, dried, and converted into sulfuric acid or oleum. This pure acid or oleum is mixed with recovered sulfuric acid and used in the digestion process.
Metal oxides produced by thermal decomposition contain all elements initially present in the raw material, except for titanium converted into pigment. This metal oxide mixture, primarily iron oxide, can be used as an additive in construction materials or the cement industry.
Increasing demand for environmentally friendly industrial processes has driven the development of techniques that can recycle the 5–30% sulfate remaining in acidic wash water. Modern processes can recover and reuse up to 99% of sulfuric acid in production.
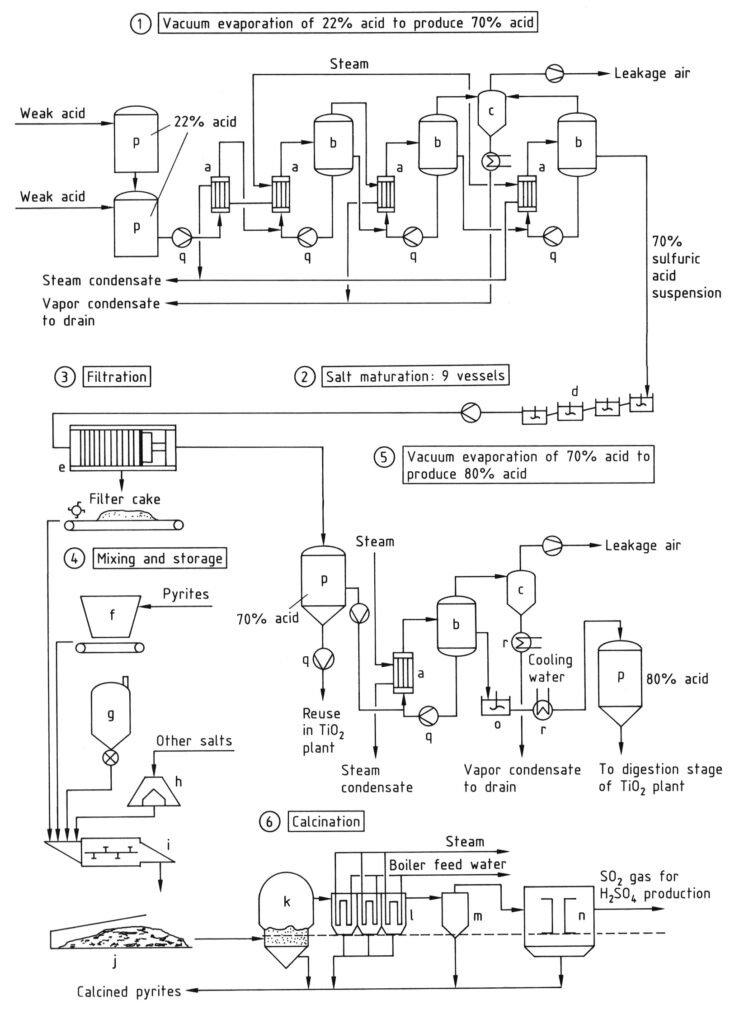
a) Heat exchanger; b) Evaporator; c) Injection condenser; d) Stirred salt maturing vessels; e) Filter press; f) Bunker for pyrites; g) Coal silo; h) Bunker; i) Mixing screw unit; j) Covered store for mixed filter cake; k) Calcination furnace; l) Waste-heat boiler; m) Cyclone; n) Electrostatic precipitator; o) Stirred tank; p) Storage tank; q) Pump; r) Cooler
4.2. Solid Waste
Solid residue from the digestion reaction is often disposed after neutralization. In the 1990s, this residue began to be used as a source of titanium in blast furnaces to stabilize the inner lining and extend blast furnace life. Other potential uses include asphalt filler (replacing limestone), landfill covering, or construction material. Some applications as an abrasive have also been suggested.
Iron sulfate heptahydrate (FeSO4·7 H2O, copperas) obtained from the crystallization and separation of black liquor or weak acid is primarily used for water purification, wastewater treatment, and as a raw material for iron oxide pigments.
Since 1980 in Scandinavia, FeSO4·7 H2O has been used as a chromate reducing agent in cement. FeSO4·H2O, monohydrate (from thermal drying of heptahydrate), has not found major use due to unfavorable performance and cost.
Metal sulfates (filter salt) recovered by filtration from weak acid after concentration (main constituent: iron(II) sulfate in pure monohydrate form) were used for many years in fertilizer manufacturing, thermal cracking for sulfuric acid production, or were landfilled.
Filter salt was first proposed as a chromate reducing agent in 2003. Subsequently, major European titanium dioxide manufacturers investigated and filed many patents on various aspects of filter salt derivative usage as chromate-reducing agents.
Filter salt has shown superior properties including rheology, heat resistance, and long-term stability, and can be directly applied into cement mills. Therefore, increasing amounts of treated filter salt (e.g., neutralized with limestone) are used in the cement industry as a Cr6+ reducing agent.
These developments, along with increasing demand for chromate-reducing agents for cement due to 2005 EU legislation changes, transformed iron sulfate wastes from an unwanted material into a commercial product.
In the chloride process, wastewater problems can arise, especially if the raw material contains less than 90% titanium dioxide. Metal chloride byproducts are sometimes disposed of in solution using deep-well injection.
Metal chloride solutions are pumped via deep boreholes into porous geological strata. Special geological formations are necessary to prevent groundwater contamination by impurities.
Increasing restrictions also apply to the chloride process. Efforts have been made to use iron chloride byproduct, for example, in water treatment and as a flocculation agent. A process in which metal chlorides are treated with cement and alkaline compounds to produce rock-like aggregates for road building has been proposed.
Another method is converting iron chloride into iron oxide via the Ruthner process, with hydrochloric acid recovery. Until recently, many chloride process facilities had to neutralize their waste metal chlorides, followed by disposal of the resulting iron hydroxide.
4.3. Waste Gas
Gases from the calcination kiln are cooled in a heat exchanger. Entrained titanium dioxide pigment is removed, washed, and recycled to the process. SO2 and SO3 formed during calcination can then be scrubbed from the gases to form dilute sulfuric acid, which is recycled.
References
- Pigments, Inorganic, 2. White Pigments. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.n20_n01.pub2
- Production of titanium dioxide. – https://ena-norm.eu/wp-content/uploads/2024/04/V.pl_.3.pdf
- A new method for production of titanium dioxide pigment. – https://www.sciencedirect.com/science/article/abs/pii/S0304386X12002423
- A Review of the Production Cycle of Titanium Dioxide Pigment. – https://www.scirp.org/journal/paperinformation?paperid=46456
- Titanium Dioxide. – https://www.ncbi.nlm.nih.gov/books/NBK524874/
- Process for manufacturing titanium dioxide pigments using ultrasonication. – https://patents.google.com/patent/US9353266B2/en
- Process for manufacturing titanium dioxide. – https://patents.google.com/patent/US4288418A/en
- An updated review of industrially relevant titanium dioxide and its environmental health effects. – https://www.sciencedirect.com/science/article/pii/S2666911023000114
