Piperidine: Properties, Reactions, Production and Uses
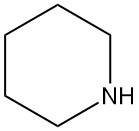
What is piperidine?
Piperidine, also known by other names such as hexahydropyridine, pentamethyleneimine, azacyclohexane, cyclopentimine, cypentil, and hexazane, is a secondary cyclic amine with the chemical formula C5H11N. It is a colorless liquid with a strong, unpleasant odor typical of amines.
Piperidine is named after piperine, which contains piperidine as part of its molecular structure as piperic acid piperidide. Piperine is the compound responsible for pepper’s flavor.
It is found in nature, especially in some alkaloids (natural compounds with medicinal properties).
Table of Contents
1. Physical Properties of Piperidine
Piperidine is a colorless liquid with an ammonia odor that is hygroscopic and fumes in the air. It is very soluble in water, lower alcohols and ketones, ethers, aliphatic and aromatic hydrocarbons, ethyl acetate, and DMF. Piperidine forms an azeotrope with water.
Table 1 summarizes some of the physical properties of piperidine.
| Property | Value |
|---|---|
| CAS number | [110-89-4] |
| Molecular formula | C5H11N |
| Molecular mass | 85.15 g/mol |
| Melting point | -10.5 °C |
| Boiling point | 106.4 °C (at 101.3 kPa) |
| Density | 0.8613 g/cm3 |
| Refractive index | 1.4532 |
| pKa | 11.1 |
| pKb at 25 °C | 2.80 |
| Vapor pressure at 25 °C | 4.0 kPa |
| Vapor Density | 3 |
| Heat of Combustion at 25 °C | 3453.2 kJ/mol (liquid) |
| Flash point | 4 °C |
2. Chemical Reactions of Piperidine
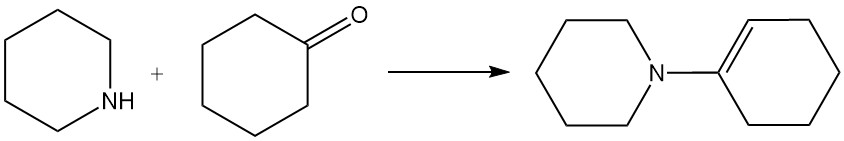
Piperidine reacts with ketones to form enamines. For example, it reacts with cyclohexanone to produce 1-(cyclohex-1-en-1-yl)piperidine.
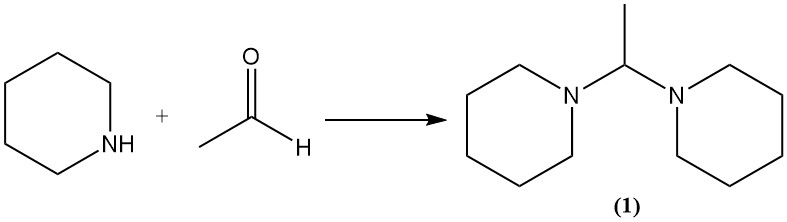
Unlike open-chain amines, piperidine reacts with aldehydes to give aminals as intermediates. As an example, it reacts with acetaldehyde to form 1,1′-(ethane-1,1-diyl)dipiperidine (1).
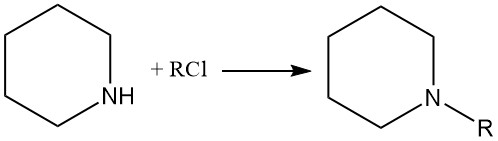
Piperidine also undergoes typical reactions of secondary amines, such as salts formation with acids, N-alkylation, amides formation, and others.
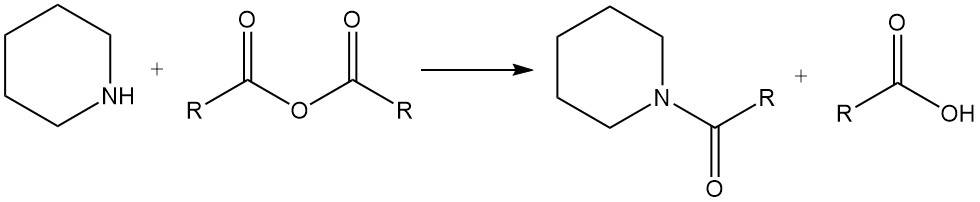
Piperidine reacts with alkyl halides and acid anhydrides to give N-alkylpiperidines and amides, respectively.
3. Production of Piperidine
Piperidine is produced by pyridine hydrogenation using platinum, palladium, or Raney nickel catalysts in liquid-phase reactions. Complete pyridine ring saturation is achievable using nickel-aluminum alloy in alkaline medium (0.5 M KOH) at ambient temperature with yields of 51 to 90%.
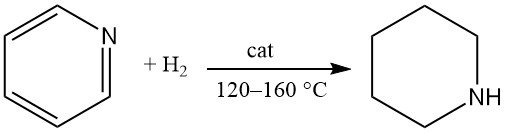
Pyridine produced from distillation of coal tar is contaminated with sulfur compounds, which need a two-stage process. First, the crude pyridine is hydrorefined using a sulfidic metal catalyst at 280–310 °C; subsequently, it is hydrogenated to piperidine using a nickel-alumina catalyst at 120–160 °C.
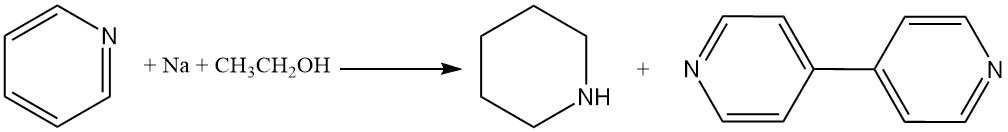
Piperidine can also be produced by treating pyridine with sodium metal in ethanol; however, the dimerization reaction produces 4,4′-bipyridine as a byproduct.
Piperidine synthesis is also possible via aminolysis of 1,5-pentanediol or tetrahydrofurfuryl alcohol under hydrogenation conditions using cobalt or nickel catalysts. Additionally, hydrogenation of glutarates, glutaric acid, or glutaraldehyde in the presence of excess ammonia yields piperidine.
4. Uses of Piperidine
Piperidine is used as a precursor for pharmaceuticals, agrochemicals, rubber additives, surfactants, and other organic compounds. Because of its high reactivity, it is frequently used as an intermediate for pharmaceuticals and for plant protection agents.
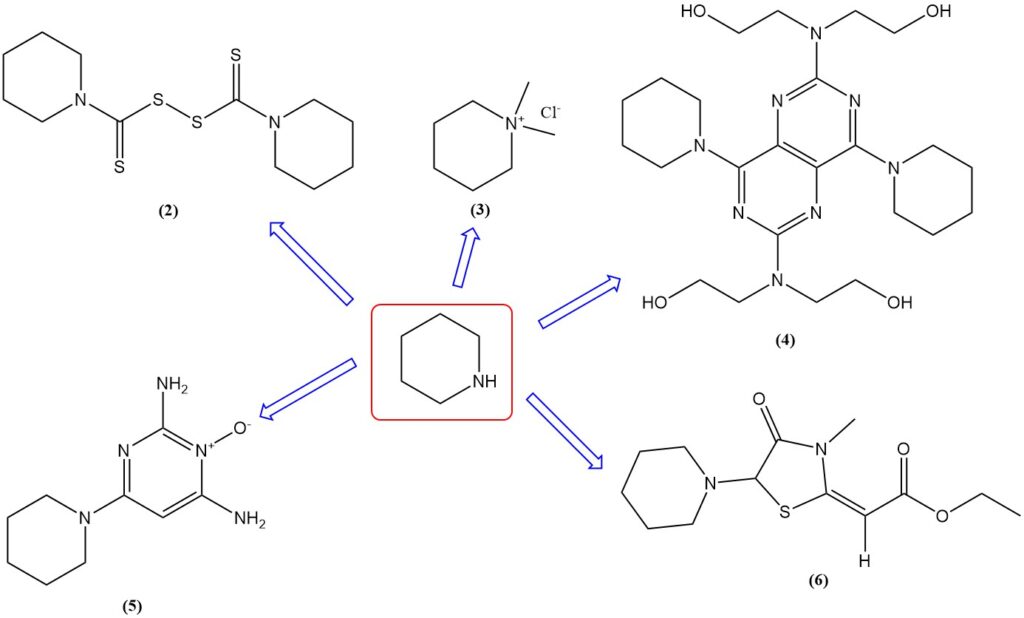
In the rubber industry, piperidine is employed to produce vulcanization accelerators like thiuram disulfide (2); and it is used as an oil or fuel additive.
Piperidine and piperidine acetate are used as catalysts in condensation reactions such as the Knoevenagel reaction, aldol condensations, and nitroparaffin-aldehyde condensations. Piperidine is preferred over stronger bases in the presence of unstable reactants or products.
Mepiquat dichloride (3), the dimethyl quaternary salt of piperidine, is used as a plant growth regulator for cotton. Piperidine is used to make vasodilators such as dipyridamole (4) and minoxidil (5) and diuretics like etozoline (6). It is also used as solvent.
Piperidine and certain piperidine salts are regulated within the United States by the Drug Enforcement Agency.
5. Toxicology of Piperidine
Piperidine induces acute dermal toxicity, characterized by severe irritation and burns. Ocular exposure results in severe irritation and potential corneal damage. Inhalation can cause respiratory tract irritation, manifesting as coughing and wheezing.
Gastrointestinal disturbances, including nausea, vomiting, salivation, and abdominal pain, may occur following exposure to piperidine. Additionally, piperidine can cause neurological symptoms such as headache, dizziness, muscle weakness, fatigue, depression, and irritability.
Long term studies on piperidine show no evidence of carcinogenicity in animal studies. Reproductive toxicity data indicate a potential for fetal harm. Long-term exposure may induce hepatotoxicity and nephrotoxicity.
No occupational exposure limits have been established for piperidine. However, this does not mean that this substance is not harmful. It should be noted that piperidine can be absorbed through your skin, thereby increasing your exposure.
Piperidine is a hazardous substance requiring stringent safety measures. To minimize exposure and potential harm, implement the following:
- Wear appropriate personal protective equipment, including gloves, eye protection, and protective clothing.
- Ensure adequate ventilation in work areas.
- Maintain good personal hygiene, including frequent handwashing.
- Provide eyewash stations and safety showers.
- Store piperidine in a cool, dry, and well-ventilated area, away from incompatible substances.
- Use proper handling techniques to prevent spills and leaks.
- Have a prepared spill response plan.
- Dispose of piperidine and contaminated waste according to regulations.
- Provide comprehensive training to personnel on hazards, handling, and emergency procedures.
References
- Amines, Aliphatic, Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/10.1002/14356007.a02_001.pub2
- Pyridine and Pyridine Derivatives. – https://onlinelibrary.wiley.com/doi/10.1002/0471238961.1625180919031809.a01.pub2
- https://onlinelibrary.wiley.com/doi/10.1002/14356007.a22_399
- https://www.sciencedirect.com/science/article/abs/pii/B9780123852359000059
- https://nj.gov/health/eoh/rtkweb/documents/fs/1543.pdf
- https://pubchem.ncbi.nlm.nih.gov/compound/Piperidine
