Boron Nitride: Properties, Production and Uses
Boron nitride (BN) is a compound formed by the 1:1 union of boron and nitrogen (elemental neighbors of carbon in the periodic table). Much like carbon, boron nitride exists in multiple crystalline forms, known as allotropes, each mirroring the structure of a specific carbon allotrope.
- α-BN: Hexagonal modification with a layered structure similar to graphite, sometimes called “white graphite.”
- β-BN: High-pressure diamond-like modification with a cubic zinc blende structure.
- γ-BN: Dense hexagonal modification with a wurtzite structure.
Table of Contents
1. Properties of Boron Nitride
Boron nitride is a fascinating material with properties resembling both graphite and diamond, its carbon counterparts. It exists in three primary forms, each with unique characteristics and applications.
1.1. α-Boron nitride: The Graphite-Like Allotrope
The most common form of boron nitride is α-BN, a hexagonal structure similar to graphite. It has numerous attractive properties:
- Low density: 2.27 g/cm³
- High-temperature stability: Melting point exceeding 3000 °C
- Chemical inertness: Resistant to acids, molten metals, and high temperatures
- Excellent thermal conductivity: Comparable to stainless steel at cryogenic temperatures and beryllium oxide at higher temperatures
- Superior electrical insulation: Low dielectric constant and high dielectric strength
- Effective lubricant: Maintains low friction coefficient up to 900 °C
- Non-wetting: Resistant to attack by various molten materials and reactive metals
- Sinterability: Hot-pressing allows creation of dense shapes
Due to its superior high-temperature stability and inertness, α-boron nitride is widely used as a refractory ceramic. It outperforms other nitride and oxide ceramics in this regard and finds applications in various high-temperature environments.
1.2. β-Boron nitride: The Diamond-Like Allotrope
β-boron nitride, also known as Borazon, possesses properties similar to diamond due to its cubic crystal structure. It features:
- Colorless and highly insulating (in pure form)
- High Knoop hardness: Approximately 4700, exceeding most materials except diamond
- Outstanding thermal stability: Oxidation resistance up to 1400 °C, significantly higher than diamond
- Excellent abrasive properties: Effective at high temperatures due to superior stability
β-boron nitride is a valuable material for cutting tools, grinding wheels, and other abrasive applications due to its diamond-like hardness and high-temperature resistance.
1.3. γ-Boron nitride: The Metastable Allotrope
γ-Boron nitride, the wurtzite form of boron nitride, is metastable under typical fabrication conditions for β-boron nitride. It has a density close to β-boron nitride and undergoes a phase transition to the cubic form at high pressures and temperatures.
Despite its limited stability compared to other forms, γ-boron nitride holds potential for specialized applications due to its unique properties.
| Property | Hot-pressed BN | Hot isostatically pressed BN | Hot-pressed composite ceramics BN/ZrO2 |
|---|---|---|---|
| Bulk density, g/cm3 | 2.0 | 2.2 | 2.8 - 3.6b |
| Porosity, vol % | < 7 | < 1 | < 7 |
| Modulus of rupture (4-point), aMPa | 100/80 | 50 | 130/70 |
| Young's modulus,a GPa | 70/35 | 30 | 80/35 |
| Thermal conductivity, cWm-1K-1 | |||
| 20 °C | 65/45 | 50 | 35 - 25/20 - 18b |
| 400 °C | 50/30 | 40 | 31 - 21/17 - 15 |
| 700 °C | 30/20 | 30 | 28 - 18/15 - 13 |
| 1000 °C | 15/10 | 20 | 25 - 15/13 - 18 |
| Thermal expansion coefficient, α10-6 K-1 | |||
| (at 20 - 1000 °C) | 1.2/8.0 | 4.0 | 4.5 - 9.0/8.5 - 11.0b |
| Specific heat, J/gK (at 20 °C) | 0.8 | 0.8 | 0.7 |
| Electrical resistivity, Ωcm (at 20 °C) | > 1012 | > 1012 | > 1012 |
| Dielectric strength, kV/mm (at 20 °C) | > 6 | > 6 | > 6 |
b Dependent on ZrO2 ratio..
Overall, boron nitride offers a remarkable combination of properties that makes it valuable for various industries. Its diverse forms cater to specific needs, ranging from high-temperature ceramics and abrasives to advanced electronics and lubricants.
2. Production of Boron Nitride
Boron nitride, a fascinating material with remarkable properties, exhibits diverse forms depending on the synthesis process. This exploration delves into the methods for producing these forms, highlighting their unique characteristics and applications.
1. Hexagonal α-Boron nitride:
The first successful synthesis of hexagonal α-boron nitride dates back to the mid-19th century by Balmain. However, it remained a mere curiosity in the scientific world until the mid-20th century, when the development of hot-pressing techniques facilitated the production of dense, shaped α-boron nitride. Today, two primary industrial methods stand out for their efficiency:
1. Reaction of boric oxide or boric acid with ammonia: This method, often utilizing tricalcium orthophosphate as a carrier, generates crystalline α-boron nitride in the form of hexagonal platelets. A subsequent heat treatment exceeding 1500 °C under nitrogen further purifies and stabilizes the material.
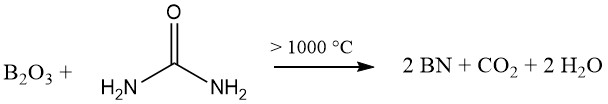
2. Reaction of boric acid or borax with organic nitrogen compounds: This approach, employing urea or melamine for instance, can yield turbostratic boron nitride. This variant exhibits a hexagonal structure but lacks complete three-dimensional order within its layers.
The quest for dense α-boron nitride led to two effective approaches:
- Axial or hot isostatic pressing: This method compresses fine powders into machinable billets, offering versatility for various applications.
- Pyrolysis of BCl3 and NH3 mixture: This process deposits α-boron nitride onto a graphite substrate, resulting in a pore-free and helium-impervious material. However, this pyrolytic form exhibits anisotropic properties due to the orientation of its layers.
Beyond these common forms, α-boron nitride can also be produced as fibers, thin films, and even intricate multidimensional composites, expanding its potential applications further.
2. Cubic β-Boron nitride:
The transformation of α-boron nitride into its cubic counterpart, β-boron nitride, also known as Borazon, requires extreme conditions: high pressures between 4 and 6 GPa coupled with temperatures ranging from 1400 to 1700 °C.
These demanding conditions are often facilitated by the presence of catalysts, such as lithium or magnesium nitride. Later advancements introduced the ternary compound Ca3B2N4 as a catalyst, enabling the production of exceptionally pure, large β-boron nitride crystals.
While the synthesis of bulk β-boron nitride crystals presents challenges, deposition techniques such as PVD and plasma CVD have emerged as viable alternatives for generating thin coatings. However, the difficulties associated with growing large crystals and depositing thick layers hinder the commercial viability of low-pressure sintering methods for β-boron nitride production.
Despite the challenges, sintering cubic boron nitride particles under high temperature and pressure, conditions where this diamond-like form is stable, has proven successful. Additionally, hot-pressing shock-synthesized γ-boron nitride at 6.7 GPa and 1600 °C leads to self-bonded compacts with a predominantly cubic structure, offering promising avenues for further exploration.
3. Wurtzite γ-Boron nitride:
The wurtzite form of boron nitride, γ-boron nitride, can be obtained by applying static compression exceeding 6.0 GPa. Alternatively, dynamic compression at 12-13 GPa below 1700 °C can also yield this form.
3. Uses of Boron Nitride
Boron nitride is used in various fields. Here’s a breakdown of the diverse uses of α-boron nitride powder, hot-pressed shapes, and the other boron nitride forms:
1. α-Boron nitride Powder and Hot-Pressed Shapes:
- Chemistry: Used as a catalyst support, high-temperature crucible material, and component for laboratory equipment.
- Metallurgy: Employed in refractory linings for furnaces, crucibles for melting reactive metals, and degassing nozzles.
- High-temperature technology: Used as heat shields, components in rocket nozzles, and insulation materials.
- Electrotechnics: Employed as insulators in high-voltage equipment and spark plugs.
- Electronics: Utilized as substrates for integrated circuits and components in high-power electronic devices.
2. α-Boron nitride Coatings:
- Employed for surface protection against wear and oxidation at high temperatures.
- Used as a lubricant for bearings and other mechanical components.
3. Cubic β-Boron nitride (Borazon):
- Primarily used for cutting, drilling, and grinding hard materials like diamond, ceramics, and hard steels.
- Offers superior chemical resistance and greater toughness compared to diamond in some applications.
4. Sintered β-Boron nitride Compacts:
- Employed as cutting tools for hard steels, nickel-based superalloys, and rock drills.
- Used as wire drawing dies due to their durability and wear resistance.
5. Wurtzite γ-Boron nitride:
- Offers potential as a lubricant due to its transformation to α-boron nitride during cutting, providing lubrication and enhancing tool life.
The Annual world production of α-boron nitride powder is approximately 1000 tons and the price of α-boron nitride powder ranges from 30 to 100 USD per kilogram.
Reference
- Boron Carbide, Boron Nitride, and Metal Borides; Ullmann’s Encyclopedia of Industrial Chemistry. – https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.a04_295.pub2
